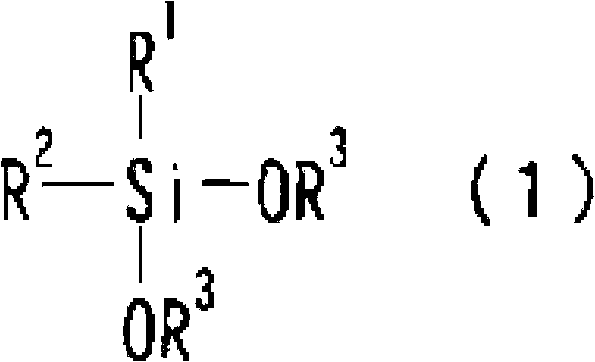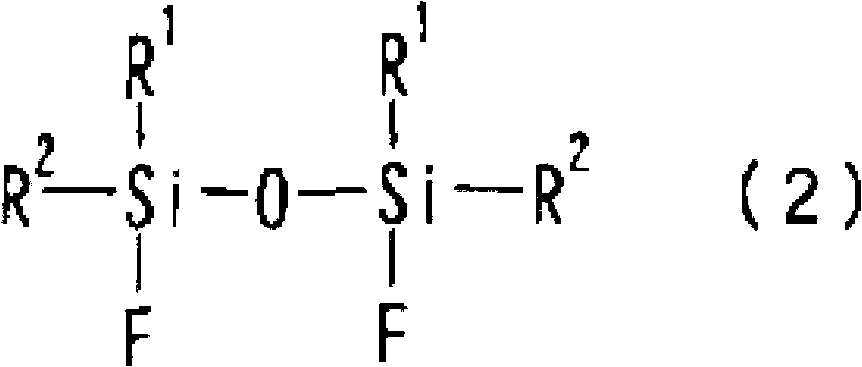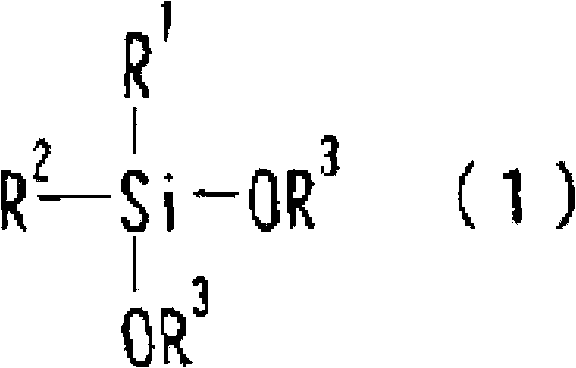1,3-difluoro disiloxane compound manufacturing method
A technology of difluorodisiloxane and manufacturing method, which is applied in the fields of compounds, chemical instruments and methods, organic chemistry, etc. of elements of group 4/14 of the periodic table, and can solve the problem of low yield of alkylchlorosilane compounds and the manufacturing method Unknown and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Put 293 g (2 moles) of dimethyldimethoxysilane into a reaction vessel equipped with a stirrer, a thermometer, and a reflux device under a nitrogen atmosphere, stir at 0°C, and simultaneously add 267 g of 15% hydrofluoric acid dropwise over 1 hour (2.2 mol as hydrogen fluoride), after the dropwise addition, stirring was further continued at 0° C. for 30 minutes. Then, the temperature was raised to 60° C. over 30 minutes, and further stirred at 60° C. for 60 minutes. 48 g of dibutyl ether was put into the reaction vessel, stirred and left to stand, the water layer was removed, the remaining organic layer was transferred to a separatory funnel and washed with 60 ml of saturated brine, and then the organic layer was dried over anhydrous sodium sulfate. The organic layer was distilled off to obtain 119 g of 1,3-difluoro-1,1,3,3-tetramethyldisiloxane (70% yield).
Embodiment 2
[0038]
[0039] In the same reaction vessel as in Example 1, under a nitrogen atmosphere, 182.5 g (1 mole) of 3-chloropropylmethyldimethoxysilane and 120 g of methanol were added, stirred at 0°C, and dropped over 1 hour. After adding 65 g of 46% hydrofluoric acid (1.2 mol as hydrogen fluoride) dropwise, stirring was further continued at 0° C. for 30 minutes. Then, the temperature was raised to 60° C. over 30 minutes, and further stirred at 60° C. for 60 minutes. 150 g of hexane and 150 g of saturated brine were put into the reaction vessel, stirred and left to stand, the water layer was removed, the remaining organic layer was transferred to a separatory funnel and washed with 60 g of saturated aqueous sodium bicarbonate solution, and then the organic layer was washed with water and sodium sulfate to dry. The organic layer was distilled off to obtain 121 g of 1,3-bis(3-chloropropyl)-1,3-difluoro-1,3-dimethyldisiloxane (82% yield).
Embodiment 3
[0041]
[0042] In the same reaction vessel as in Example 1, under a nitrogen atmosphere, 182.5 g (1 mole) of phenylmethyldimethoxysilane and 120 g of methanol were added, stirred at 0°C, and 23% After adding 130 g of hydrofluoric acid (1.2 mol as hydrogen fluoride) dropwise, stirring was further continued at 0° C. for 30 minutes. Then, the temperature was raised to 60° C. over 30 minutes, and further stirred at 60° C. for 60 minutes. 150 g of hexane and 150 g of saturated brine were put into the reaction vessel, stirred and left to stand, the water layer was removed, the remaining organic layer was transferred to a separatory funnel and washed with 60 g of saturated aqueous sodium bicarbonate solution, and then the organic layer was washed with water and sodium sulfate to dry. The organic layer was distilled off to obtain 106 g of 1,3-difluoro-1,3-diphenyl-1,3-dimethyldisiloxane (72% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com