Controlled release pellet preparation and its preparation method
A technology of sustained-release pellets and sustained-release layers, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effects of good release uniformity, good drug release, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
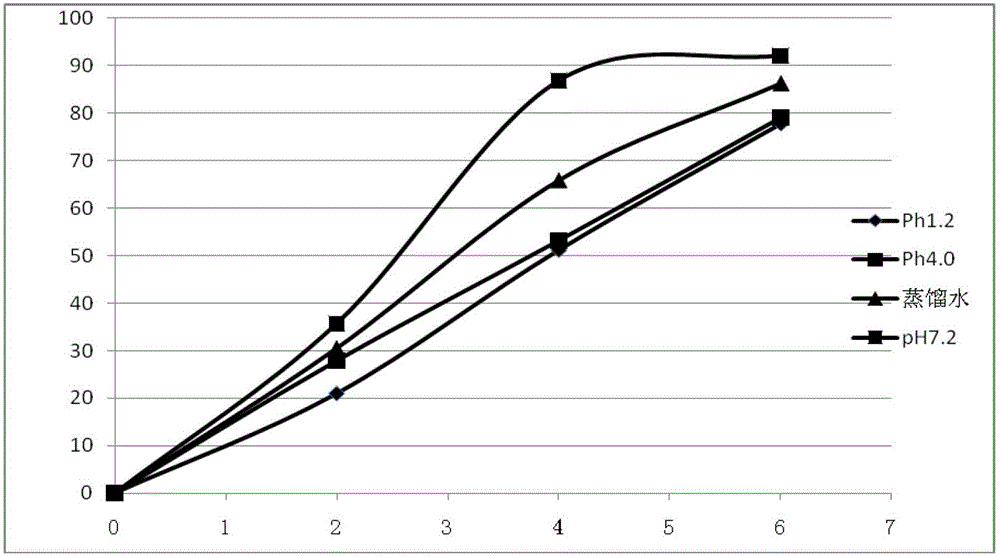
Image
Examples
Embodiment 1
[0134] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0135] 1) Sucrose core 605g
[0136] 2) Drug-loaded slow-release layer
[0137] Tamsulosin Hydrochloride 1g
[0138] Cellulose acetate 33.75g
[0139] Tween 80 7.5g
[0140] 3) Coating layer
[0141] Udrake L100-55 11.25g
[0142] Sodium Lauryl Sulfate 7.5g
[0143] Weigh the prescribed amount of 2) each component in the drug-loaded sustained-release layer, and dissolve it in about 600 g of 95% ethanol solution to prepare the drug solution.
[0144] Weigh 3) each component of the coating layer and about 500 g of purified water, and prepare the coating liquid with the above auxiliary materials.
[0145] Put the blank pellet core in the fluidized bed, control the temperature of the material at 30-35°C, adjust the fluidized state, apply the medicine for about 2 hours, dry for 10 minutes after the medicine is applied, take out the upper pill and sieve, the mesh size of the sieve is 18-30 mesh, tak...
Embodiment 2
[0148] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0149] 1) Sucrose core 505g
[0150] 2) Drug-loaded slow-release layer
[0151]
[0152] 3) Coating layer
[0153]
[0154]
[0155] Preparation
[0156] Step 1: Weigh each component according to the formula, dissolve each component of the drug-loaded sustained-release layer in about 500g of 95% ethanol to prepare a drug solution, apply the drug in a fluidized bed, and control the temperature of the material at 30-55°C to prepare on the pill.
[0157] Step 2: Sieve the upper pills with a mesh number of 15-30 mesh.
[0158] Step 3: Take about 550g of ethanol to dissolve the components of the coating layer; place the sieved upper pills in a fluidized bed, control the temperature of the material at 45-55°C, and continue drying for 15 minutes after the coating is completed.
[0159] Step 4: Add about 1 / 2 the amount of talcum powder to 3 times the amount of water, spray it in after homogenizi...
Embodiment 3
[0162] Tamsulosin Hydrochloride Sustained Release Capsules (5000 capsules)
[0163] 1) Sucrose core 660g
[0164] 2) Drug-loaded slow-release layer
[0165] Tamsulosin Hydrochloride 10g
[0166] Sodium Alginate 300g
[0167] 3) Coating layer
[0168] Udrake L100-55 10g
[0169] TEC 5g
[0170] 4) Micropowder silica gel 4g
[0172] Preparation
[0173] Step 1: Weigh each component according to the formula, dissolve each component of the drug-loaded slow-release layer in about 300g ethanol to prepare a drug solution, apply the drug in a fluidized bed, and control the temperature of the material at 40-45°C to prepare the drug solution. pill.
[0174] Step 2: Add micropowder silica gel to twice the amount of water, spray it in after homogenizing with a high-speed homogenizer for 5 minutes, dry it for 5 minutes, and take it out.
[0175] Step 3: Sieve the upper pills with a mesh number of 15-30 mesh.
[0176] Step 4: Take about 100g of ethanol to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com