New emd formulation comprising pga
A preparation, surface stabilizer technology, applied in the field of new EMD preparations containing PGA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Weight average molecular weight ( m W )
[0135] The weight average molecular weight was determined according to analytical method AM-S01 using GPC (Agilent) and the following parameters:
[0136] Eluent: Phosphate buffer pH 7.0
[0137] Precolumn: PSS Suprema 10 μm 100 ? 8 x 50 mm
[0138] PSS Suprema 10 μm 100 ? 8 x 300 mm
[0139] PSS Suprema 10 μm 1000 ? 8 x 300 mm
[0140] PSS Suprema 10 μm 100 ? 8 x 300 mm
[0141] Pump: Agilent 1100
[0142] Flow rate: 1.0 ml / min
[0143] Autosampler: Agilent 1100, injection volume 50 μl
[0144] Sample concentration: 2.0, 3.0 g / L
[0145] Temperature: 23°C
[0146] Detector: Agilent 1100 Rl
[0147] Calculation: PSS WinGPC Unity Version 7.20
[0148] Calibrate according to routine calibration with Pollulan molar mass standards
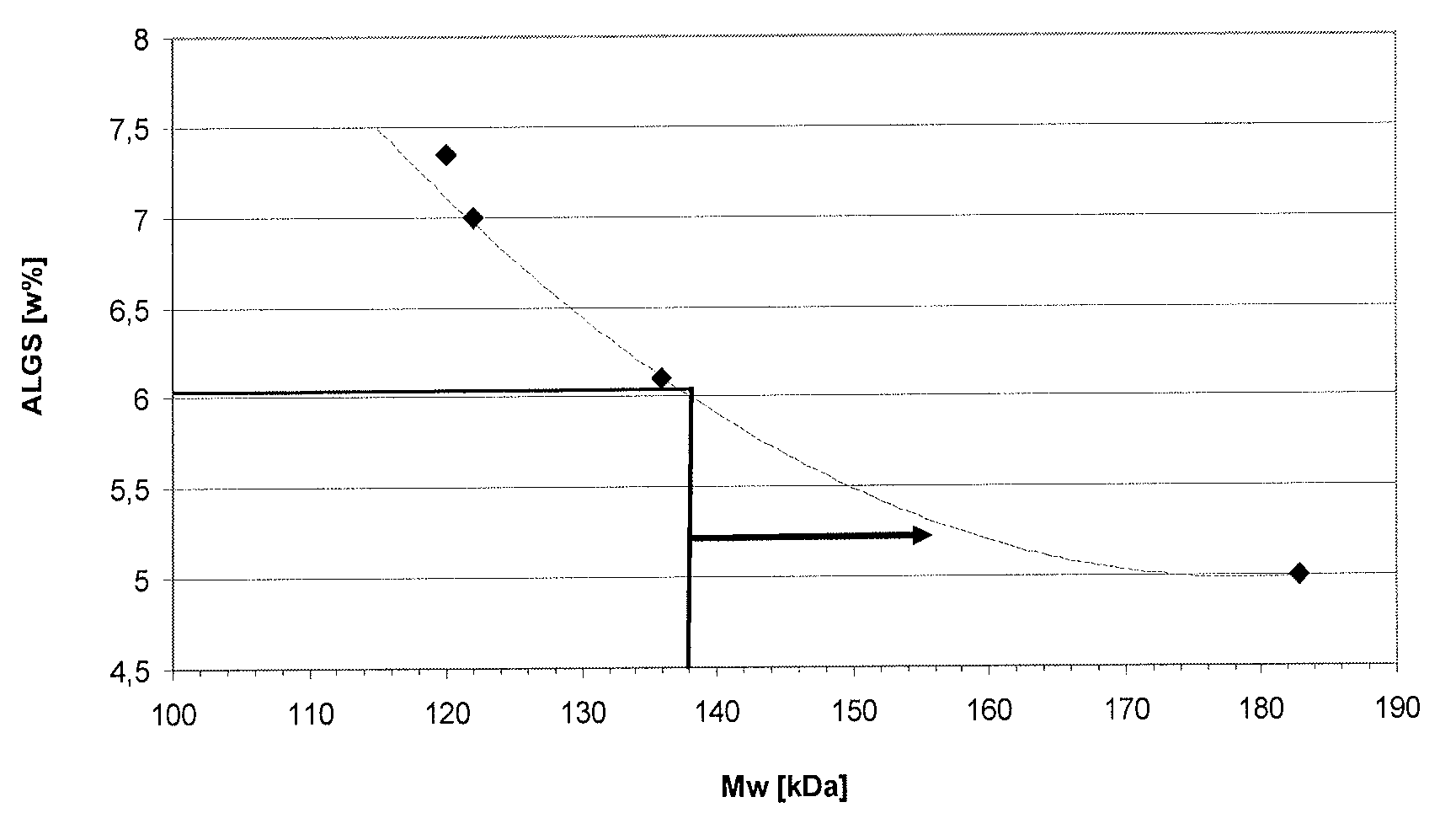
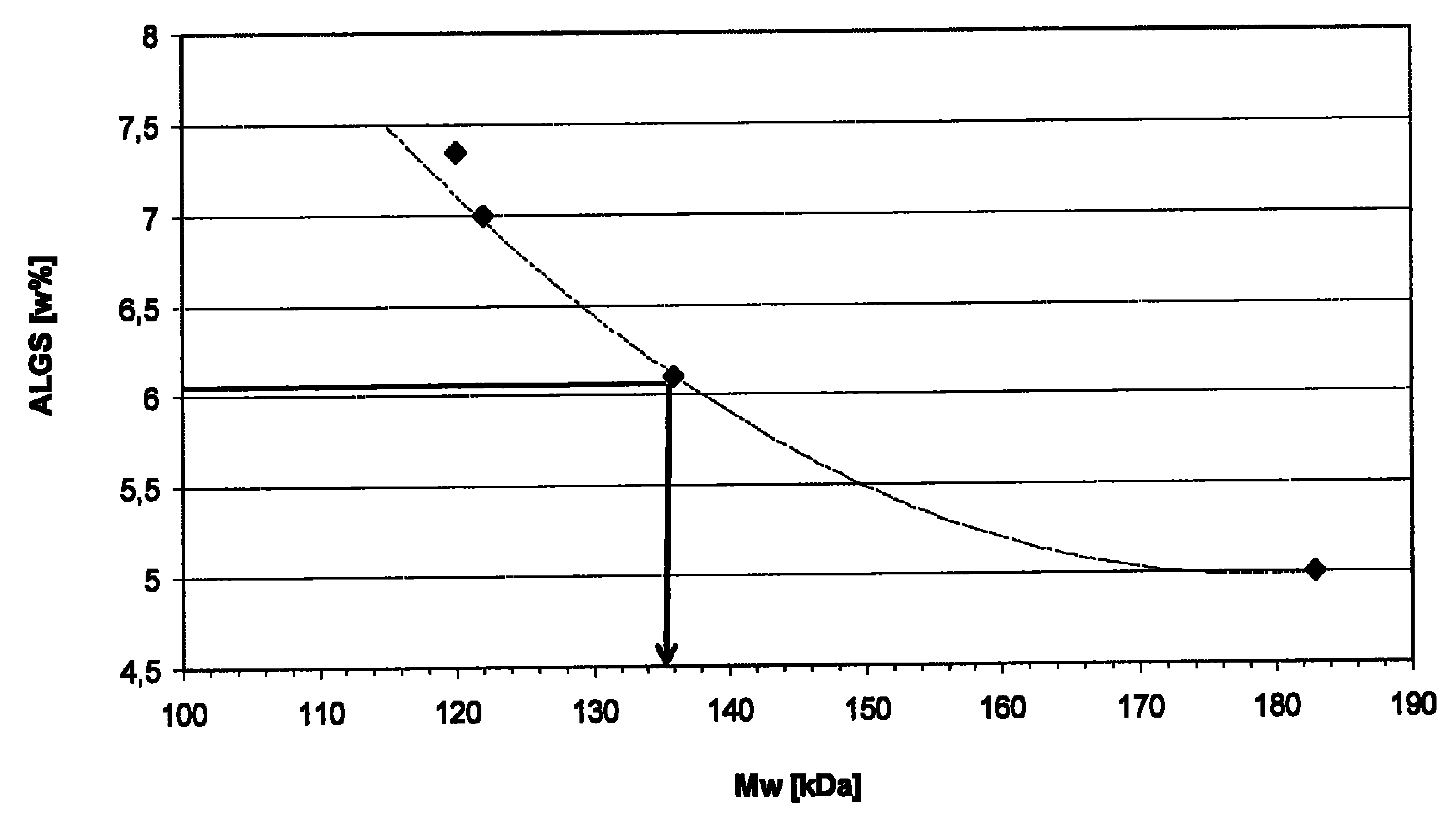
[0149] Unsterilized PGA and Kelcoloid since 2002 to 2009 ? The weight average molecular weight of O is disclosed in Table 1.
[0150] Table 1
[0151]
[0152] The table shows that ...
Embodiment 2
[0154] Sterilized PGA for Emdogain formulations was obtained from electron beam sterilization of non-sterile PGA as recommended by ISO 1137-2 (2006). The sterilization method includes the following steps:
[0155] 1. Sterility Assurance Level (SAL) 10 -6 (one milliomod) applied to 1 sterilized unit of PGA (100 g PGA / bag)
[0156] 2. Calculate the total bioburden of the sterilization unit (100 g PGA / bag): total bioburden = 100 g * CFU / g --> if the sterilization unit is changed, for example, the package is only 50 g PGA, then the total bioburden reduce
[0157] 3. Therefore, apply the total bioburden VDmax 25 --> min 25 kGy
[0158] During electron beam irradiation, PGA does not undergo crosslinking. The main process induced by electron beam irradiation is chain scission. Therefore, in this case, the weight average molecular weight (M W ) as a function of electron beam dose (R) can be simplified by Charlesby's equation (Eq. 1). The starting weight average molecular weigh...
Embodiment 3
[0166] PGA degradation.
[0167] They identified two types of degradation:
[0168] 1) Provides hydrolysis (pH change) of ester bonds of carboxylic acid and propylene glycol
[0169] 2) Degradation of glycosidic linkages in the polysaccharide backbone with reduced viscosity
[0170] They found that under acidic conditions, the ester groups in PGA were stable to hydrolysis and only the hydrolytic degradation of the polysaccharide backbone occurred. However, this statement was not confirmed under moderately acidic conditions (about pH 3-5). Hydrolytic cleavage of the polymer backbone occurs simultaneously with ester hydrolysis, and both pH and viscosity decrease.
[0171] The overall rate of hydrolysis of an ester can be written as the sum of the individual rates of the individual acid-catalyzed rates: k A [ester][ H + ], neutral rate: [Ester] and base-catalyzed hydrolysis rates: k B [ester][ Oh - ]
[0172]
[0173] in is the reaction rate constant, [ester], ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com