Supported bimetal nanocrystal catalyst and preparation method thereof
A bimetallic nano-catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of poor activity and stability, easy segregation, alloy low degree of alloying, etc., to achieve the effect of simple preparation method, improved catalyst selectivity, and improved alloying degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
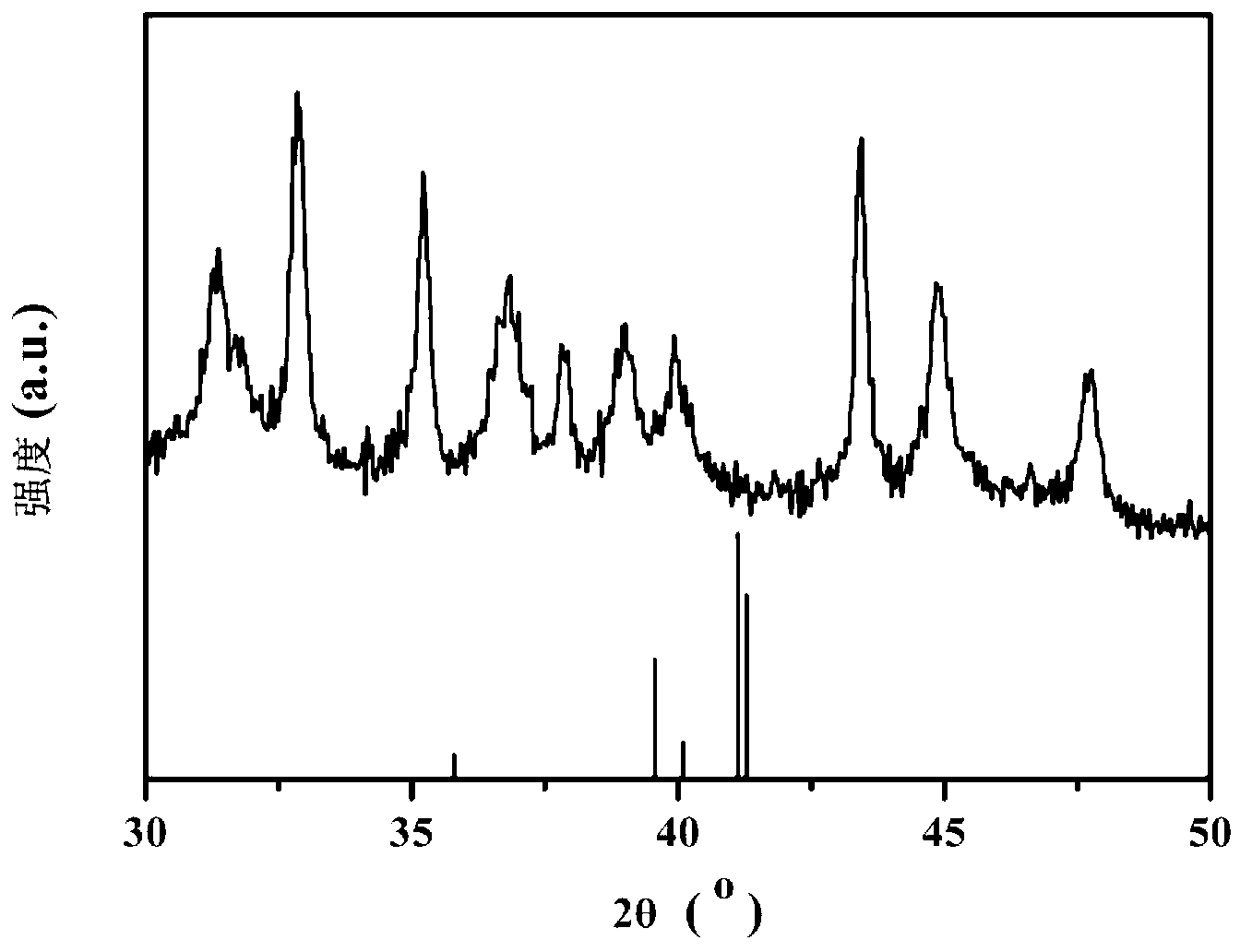
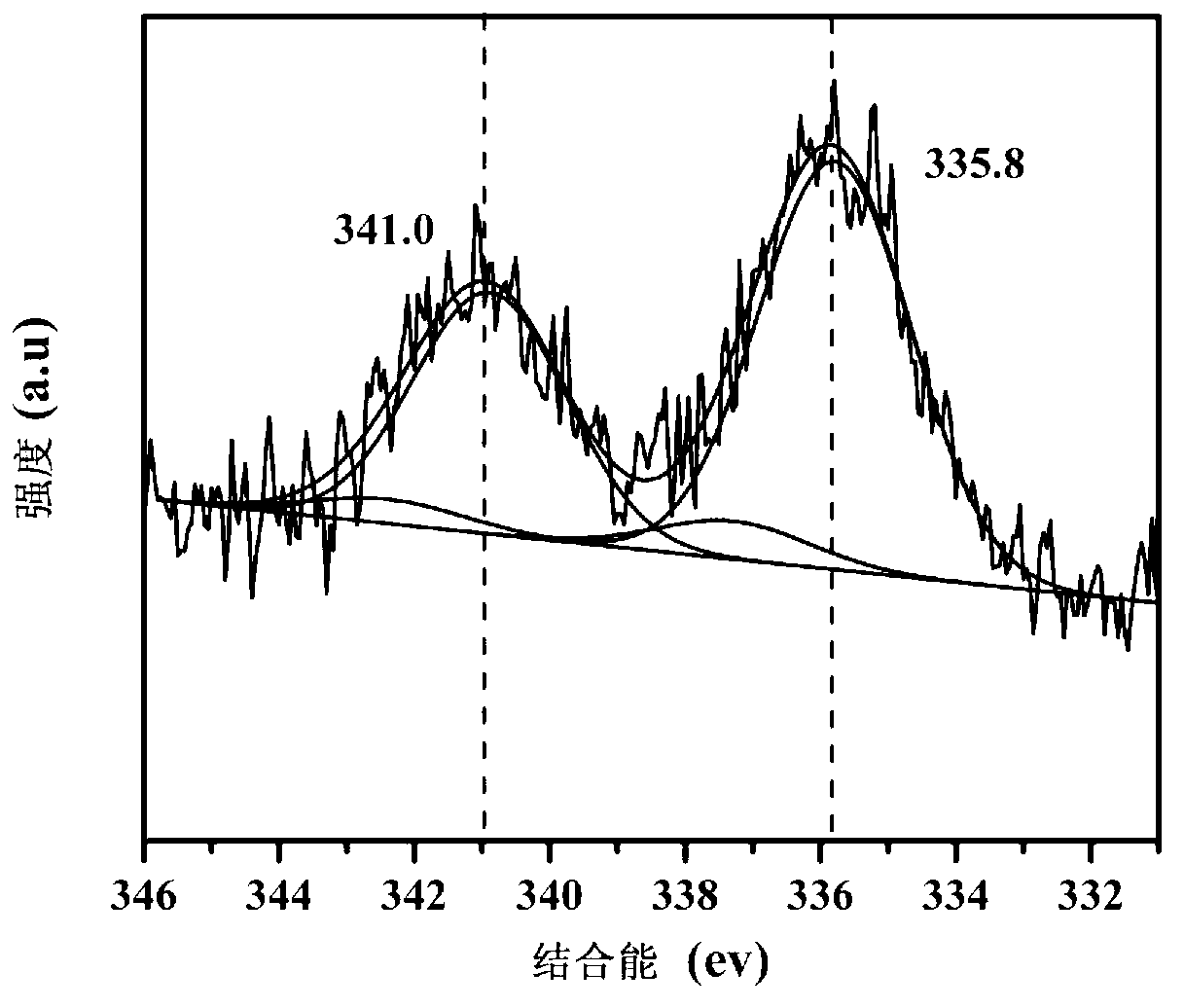
Image
Examples
Embodiment 1
[0032] A. Weigh 0.6800gMg(NO 3 ) 2 ·6H 2 O, 0.0012gGa(NO 3 ) 3 ·xH 2 O was dissolved in 50mL deionized water to prepare a metal salt solution and stirred evenly;
[0033] B, take by weighing 1.1449g urea and be dissolved in the prepared mixed salt solution of step A, stir;
[0034] C. Transfer the above-mentioned mixed solution to a hydrothermal kettle, weigh 2.0000g of spherical alumina calcined at 1150°C and add it, and conduct a crystallization reaction at 130°C for 6 hours. The obtained product is washed until the pH value of the supernatant = 7, and the solid particles are dried in an oven at 70°C to obtain MgGaAl-LDHsAl 2 o 3 sample;
[0035] D. Weigh 0.0011g NaCl and 0.0017g PdCl at a molar ratio of 2:1 2 Dissolved in 10ml of deionized water, prepared as metal Pd precursor Na 2 PdCl 4 Solution; Weigh 2.0000g step C gained precursor, place in Na 2 PdCl 4 Soak in the solution, shake once every five minutes, pour out the remaining solution after one hour of im...
Embodiment 2
[0039] A. Weigh 0.6800gMg(NO 3 ) 2 ·6H 2 O, 0.0121g Ga(NO 3 ) 3 ·xH 2 O was dissolved in 50mL deionized water to prepare a metal salt solution and stirred evenly;
[0040] B, take by weighing 1.1449g urea and be dissolved in the prepared mixed salt solution of step A, stir;
[0041] C. Transfer the above-mentioned mixed solution to a hydrothermal kettle, weigh 2.0000g of spherical alumina calcined at 1150°C and add it, and conduct a crystallization reaction at 130°C for 6 hours. The obtained product is washed until the pH value of the supernatant = 7, and the solid particles are dried in an oven at 70°C to obtain MgGaAl-LDHsAl 2 o 3 sample;
[0042] Step D is the same as embodiment 1
[0043] E. Place the catalyst precursor obtained in step D in an atmosphere furnace for high-temperature roasting and reduction, and the reducing gas is 10% H 2 / Ar, control the heating rate to 2°C min -1 , heated up to 550°C for 6 hours, cooled naturally to obtain PdGa 5 / MgO-Al 2 o ...
Embodiment 3
[0046] A. Weigh 0.6800gMg(NO 3 ) 2 ·6H 2 O, 0.0024g Ga(NO 3 ) 3 ·xH 2 O was dissolved in 50mL deionized water to prepare a metal salt solution and stirred evenly;
[0047] B, take by weighing 1.1449g urea and be dissolved in the prepared mixed salt solution of step A, stir;
[0048] C. Transfer the above-mentioned mixed solution to a hydrothermal kettle, weigh 2.0000g of spherical alumina calcined at 1150°C and add it, and conduct a crystallization reaction at 130°C for 6 hours. The obtained product is washed until the pH value of the supernatant = 7, and the solid particles are dried in an oven at 70°C to obtain MgGaAl-LDHsAl 2 o 3 sample;
[0049] Step D is the same as embodiment 1
[0050] E. Place the catalyst precursor obtained in step D in an atmosphere furnace for high-temperature roasting and reduction, and the reducing gas is 10% H 2 / Ar, control the heating rate to 2°C min -1 , heated up to 550°C for 6 hours, then cooled naturally to obtain PdGa / MgO-Al 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com