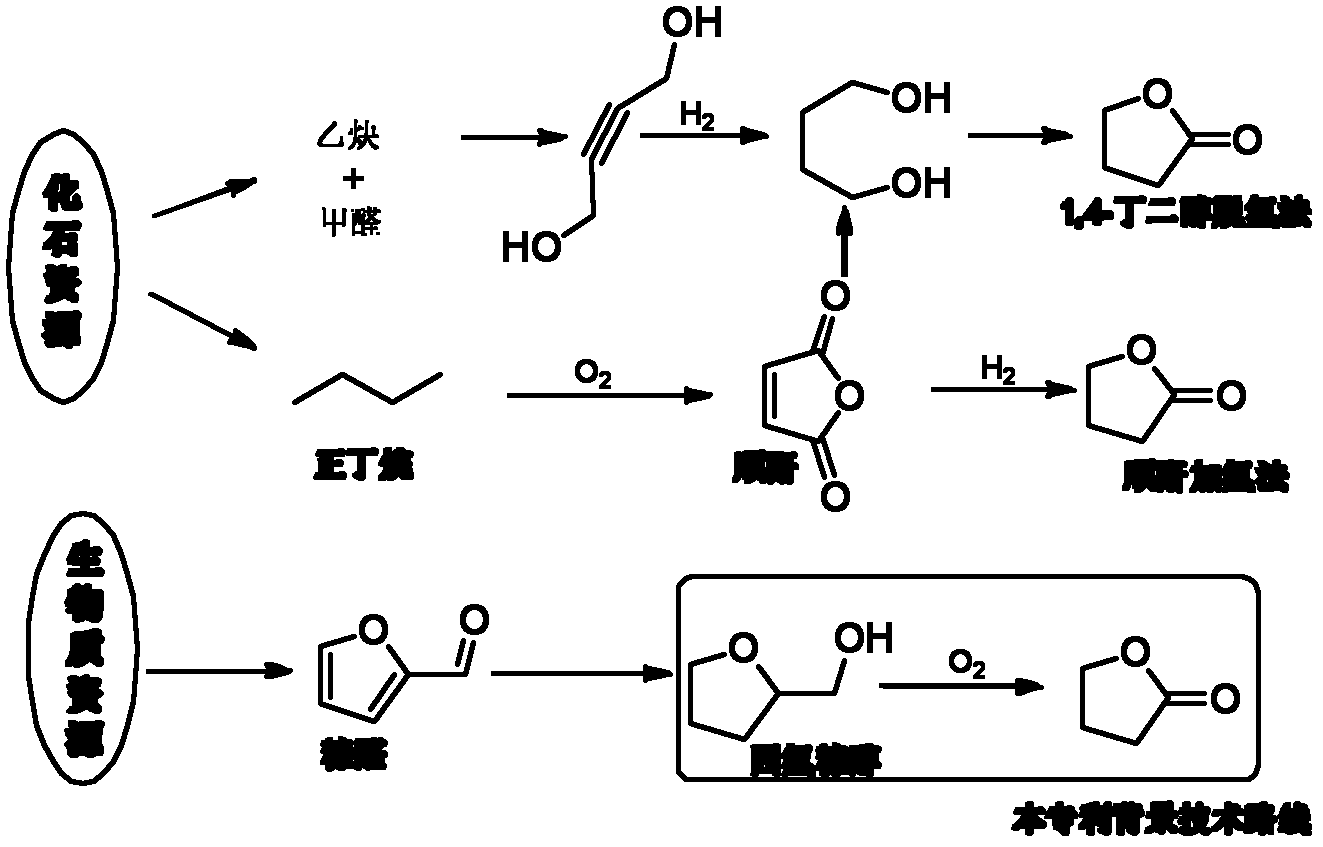
Preparation method of gamma-butyrolactone
A technology of butyrolactone and vanadyl, which is applied in the field of preparation of γ-butyrolactone, can solve the problems of harsh reaction conditions and large fluctuations, and achieve mild reaction conditions, abundant sources, and obvious innovative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
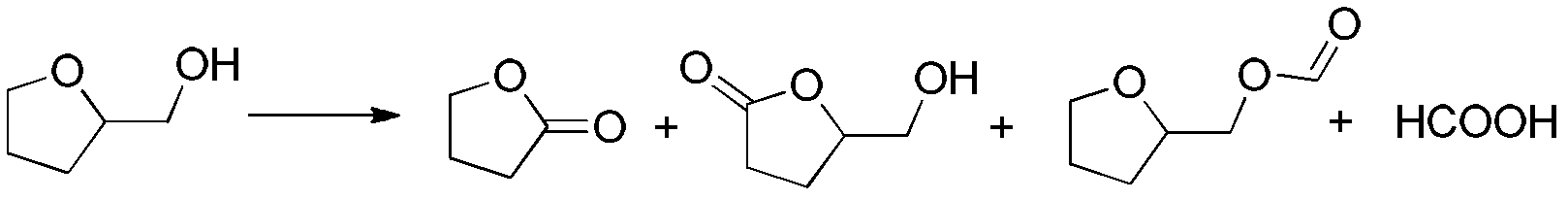
[0017] Example 1: Add 1.02g of tetrahydrofurfuryl alcohol and 1mol% vanadyl tartrate into a 50mL reaction kettle to close the kettle, fill it with oxygen at a pressure of 1.5MPa, heat up to 100°C under stirring, and run for 8h. If there is oxygen consumption during the period, is supplemented. It was then cooled to room temperature and carefully depressurized to atmospheric pressure. Sampling was used to analyze the product by GC-MS, and the chromatographic retention time of the main components of the product was compared with the γ-butyrolactone and tetrahydrofurfuryl alcohol standards to determine the main product. Product quantitative analysis uses gas chromatography (GC), tetrahydrofurfuryl alcohol transformation rate 96%, gamma-butyrolactone selectivity is 74%, other products such as figure 2 shown
Embodiment 2
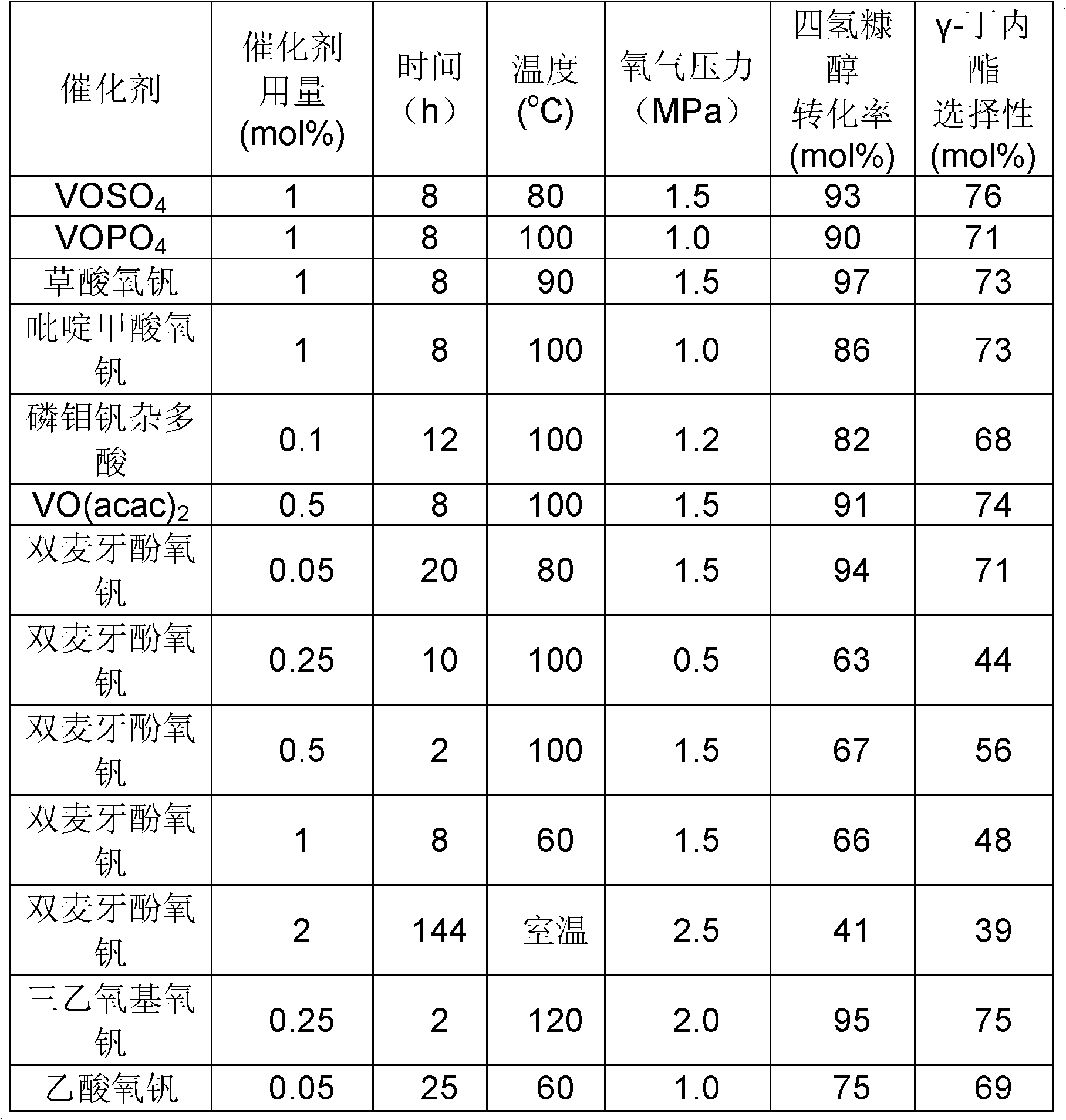
[0018] Embodiment 2: Except that the catalyst type, catalyst consumption, reaction time, and oxygen partial pressure are different, other experimental conditions are all the same as in Example 1, and the activity evaluation of different catalysts is carried out according to the steps in Example 1. The catalytic activities of different vanadium-based catalysts are shown in Table 1:
[0019] Table 1: Preparation of γ-butyrolactone by selective oxidation of tetrahydrofurfuryl alcohol
[0020]
Embodiment 3
[0021] Example 3: 5.10 g of tetrahydrofurfuryl alcohol and 0.1 mol% vanadyl sulfate were added to a 50 mL reactor to close the reactor, filled with oxygen at a pressure of 1.0 MPa, heated to 80° C. under stirring, and kept for 15 hours. Then cooled to room temperature, decompressed to normal pressure, sampling analysis, tetrahydrofurfuryl alcohol conversion rate was 79%, gamma-butyrolactone selectivity was 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com