Method for preparing graphene through phase peeling of graphite by using mixed solvent
A technology of mixing solvent phases and exfoliating graphite, applied in the direction of graphene, nano-carbon, etc., can solve the problems of toxicity, mismatch of Hansen solubility parameters, and difficult removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
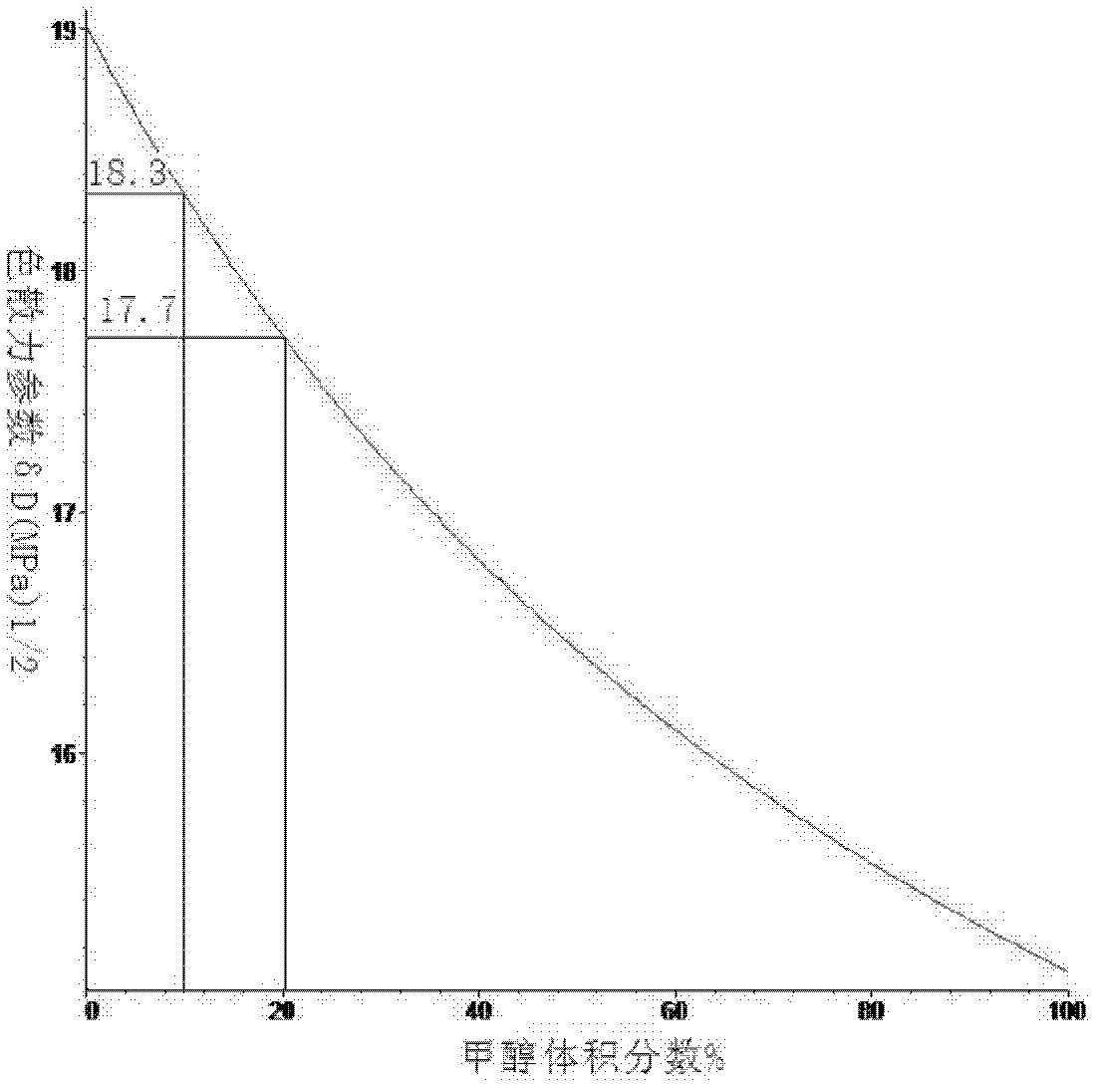
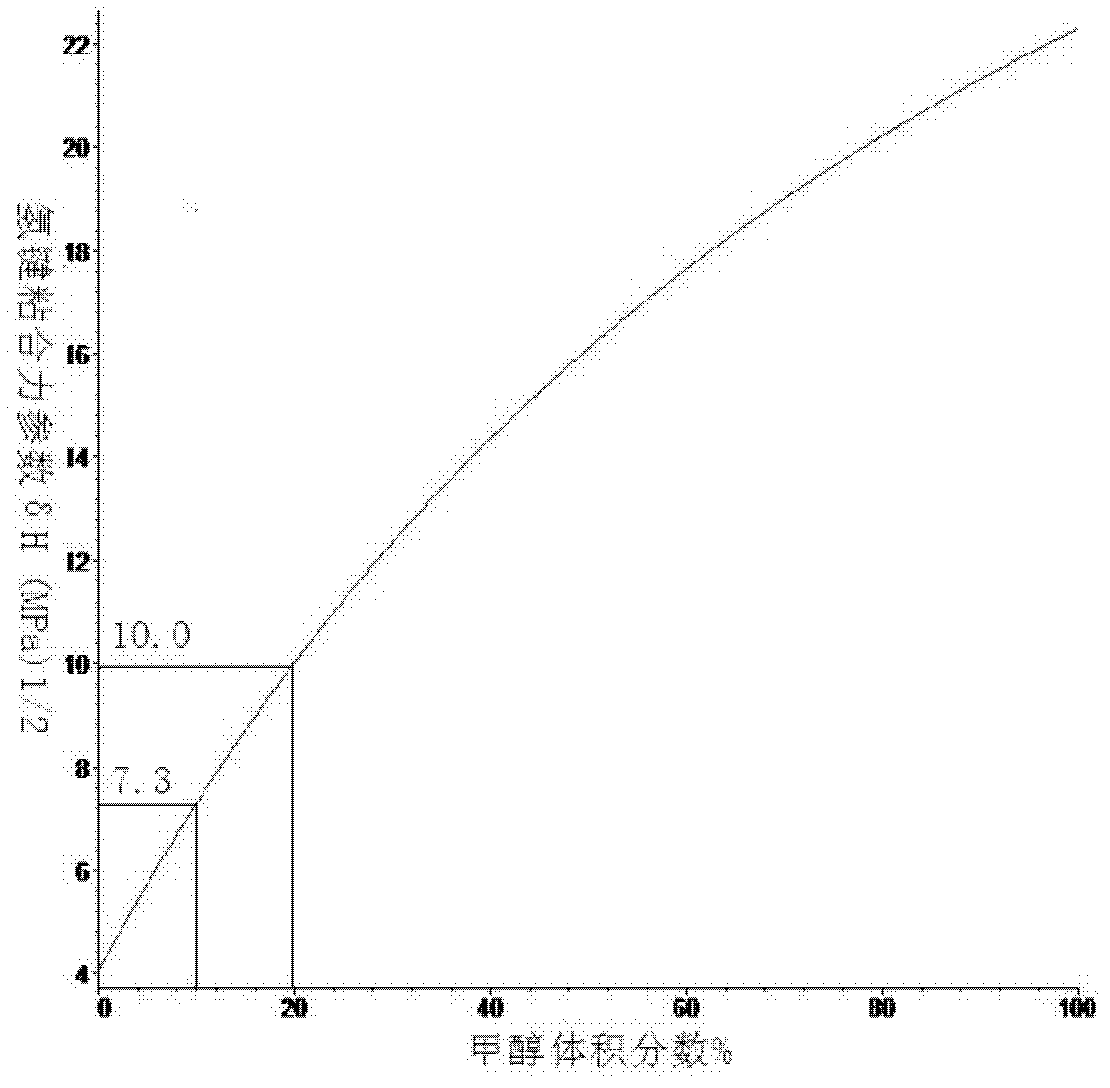
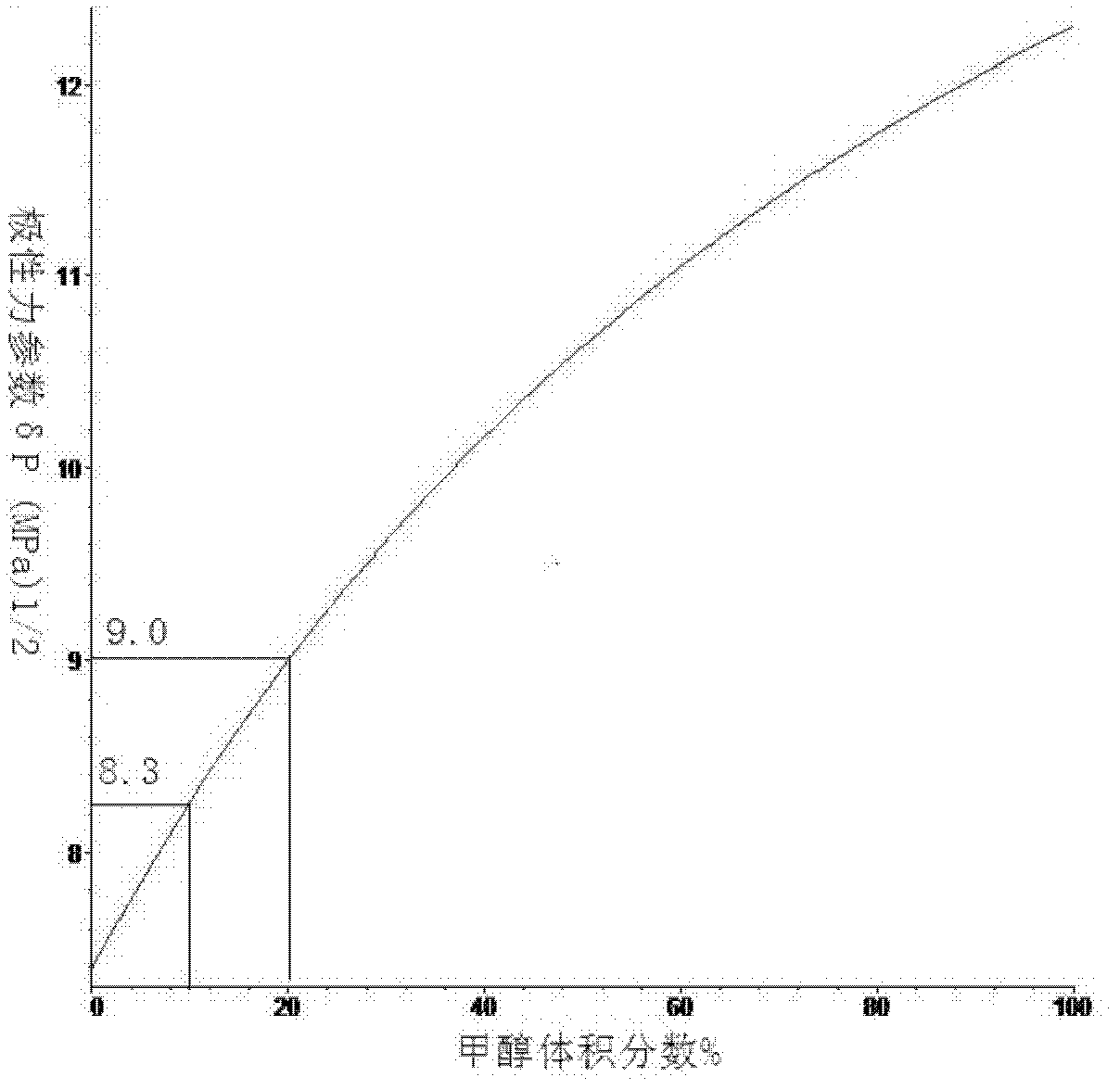
[0050] like Figure 1 to Figure 3 Shown is the curve of the Hansen solubility parameter of the mixed solvent changing with the volume fraction of methanol, according to Figure 4 Shown, calculate the optimal volume ratio when the Hansen solubility parameter of methanol and ethylene dichloride mixed solvent is close to graphene: 1, start; 2, utilize least square method to solve optimal mole fraction matrix equation: Ax=b (solve Utilize the Hansen solubility parameter 3,4 of methanol and ethylene dichloride in the process; 5, solve the equation that mole fraction is converted into volume fraction (use the relative molecular weight of methanol and ethylene dichloride in the solution process, relative density 6,7 ); 8, the solubility parameter of the mixed solvent is closest to graphene; 9, end.
[0051] Embodiment 1 calculates the optimum volume fraction of methyl alcohol and ethylene dichloride, by the Hansen solubility parameter of methyl alcohol: δ d =15.1(MPa) 1 / 2 ,δ p =1...
Embodiment 2
[0056] Embodiment 2 changes the solvent in the implementation case 1 into methanol, toluene, and the Hansen solubility parameter of methanol: δ d =15.1(MPa) 1 / 2 ,δ p =12.3(MPa) 1 / 2 ,δ h =22.3(MPa) 1 / 2 , Toluene Hansen Solubility Parameter: δ d =18(MPa) 1 / 2 ,δ p =1.4(MPa) 1 / 2 ,δ h =2(MPa) 1 / 2, replace the A matrix in Ax=b with A = 15.1 - 18 12.3 - 1.4 22.3 - 2 , b = 18 - 18 9.3 - 1.4 7.7 ...
Embodiment 3
[0061] Embodiment 3 changes the solvent in the implementation case 1 into ethanol, chloroform, the Hansen solubility parameter of ethanol: δ d =15.8(MPa) 1 / 2 ,δ p =8.8(MPa) 1 / 2 ,δ h =19.4(MPa) 1 / 2 , chloroform Hansen solubility parameter: δ d =17.8(MPa) 1 / 2 ,δ p =3.1(MPa) 1 / 2 ,δ h =5.7(MPa) 1 / 2 , replace the A matrix in Ax=b with A = 15.8 - 17.8 8.8 - 3.1 19.4 - 5.7 , b = 18 - 17.8 9.3 - 3.1 7.7 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com