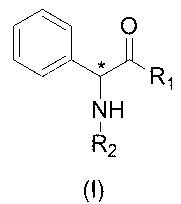
Phenylglycine histone deacetylase inhibitor as well as preparation method and applications thereof
A technology of phenylglycine and deacetylase, applied in the preparation of sulfonamides, carbamic acid derivatives, urea derivatives, etc., can solve the problems of poor pharmacokinetic properties, poor drug efficacy, and difficult absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1. Synthesis of compounds of the present invention
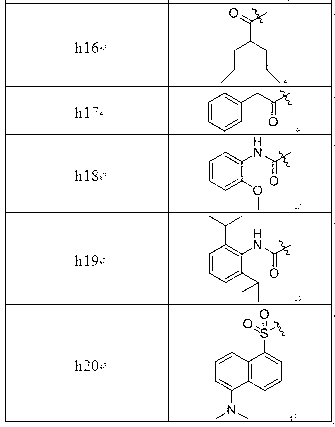
[0131] by( h2 )and( q1 ) as an example:
[0132] 1) (S)-2-((tert-butoxycarbonyl)-amino)-2-phenylacetic acid b
[0133] To a mixed solution of L-phenylglycine (15.1 g, 100 mmol) dissolved in 300 mL of methanol and water (methanol:water=9:1), triethylamine (15.1 g, 150 mmol) was added, and after 30 minutes of ice bathing, carbonic acid was added Di-tert-butyl ester (32.7 g, 150 mmol). After reacting at room temperature for 8 hours, the solvent in the reaction liquid was evaporated, acidified to pH 4-5 with 1 mol / L citric acid solution, then extracted three times with ethyl acetate, the organic phases were combined and washed with saturated brine, anhydrous sulfuric acid Dried over magnesium and evaporated to dryness to obtain a crude product, which was recrystallized from a mixed solvent of ethyl acetate and n-hexane (ethyl acetate:n-hexane=1:8) to obtain 17.2 g of a white solid. Yield: 91.56%, ESI-M...
Embodiment 2
[0162] Example 2 Target compound inhibits histone deacetylase activity test (In vitro)
[0163] The fluorescent analysis method of histone deacetylase (HDACs) activity is mainly divided into two steps: the first step, the fluorescent substrate of lysine HDACs (Boc-Lys(acetyl)-AMC) containing an acetylated side chain, is used Hela cell extract samples of protein deacetylases (including HDAC1, HDAC2, HDAC3 and HDAC8) were incubated to deacetylate and activate substrates. In the second step, trypsin is used to hydrolyze Boc-Lys-AMC to generate AMC, a fluorescent group (ie, a chromophore), and the fluorescence intensity is measured at the emission wavelength / excitation wavelength (390nm / 460nm), so that according to the inhibitor group and control Calculate the inhibition rate from the fluorescence intensity of the group, and calculate the IC 50 value. For the principle of enzyme activity test, please refer to the relevant content of this patent specification. The experimental...
Embodiment 3
[0169] Example 3 The activity test of the target compound in inhibiting cell proliferation (In vitro)
[0170] Select compounds with better enzymatic activity h8, q4, q6 The activity test of inhibiting the proliferation of cancer cells in vitro was carried out, and the results are shown in Table 2.
[0171] Terminology Explanation:
[0172] U937: Human histiocytic lymphoma cell line.
[0173] K562: human erythroleukemia cell line.
[0174] HL60: human acute leukemia cell line.
[0175] SAHA: The product name is Zolinza, and the generic name is Vorinostat. It is a histone deacetylase inhibitor approved by the US Food and Drug Administration (FDA) in 2006.
[0176] DMSO: dimethylsulfoxide.
[0177] IC 50 : half inhibitory concentration.
[0178] 1. [Materials] U937, K562, HL60 cell lines, MTT tetramethylazoblue, 10% fetal bovine serum, 96-well plate
[0179] 2. [Method]
[0180] cell culture U937, K562, and HL60 three tumor cell lines were cultured conventionally....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com