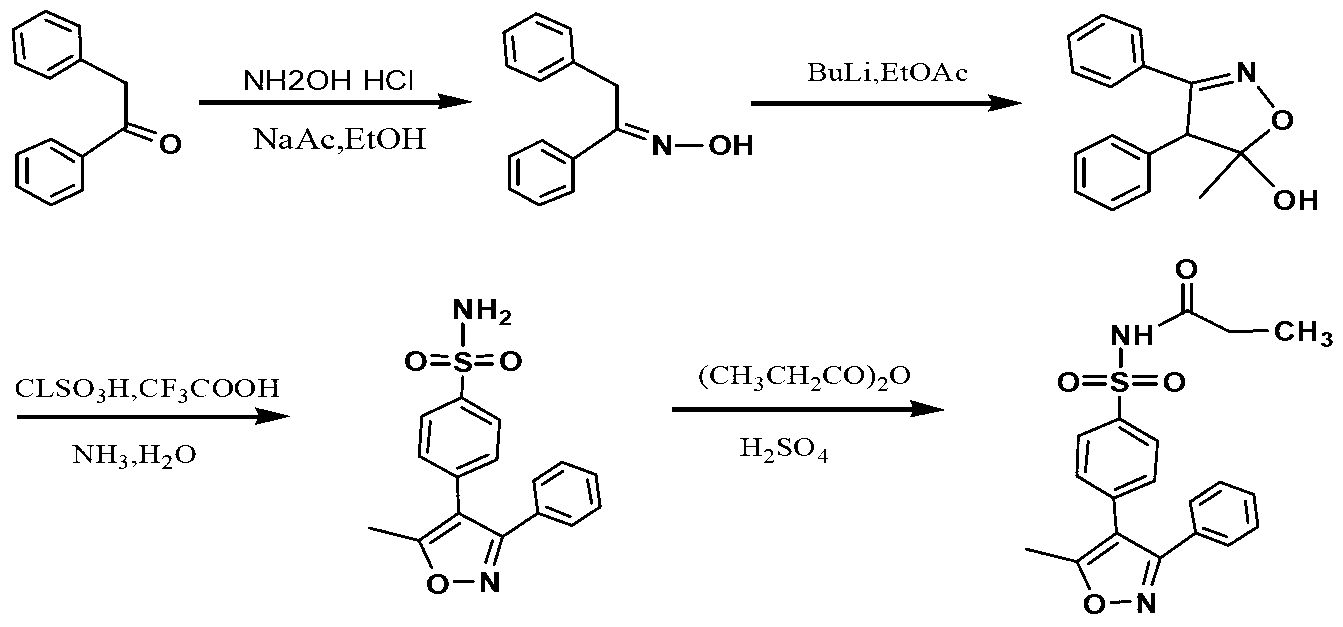
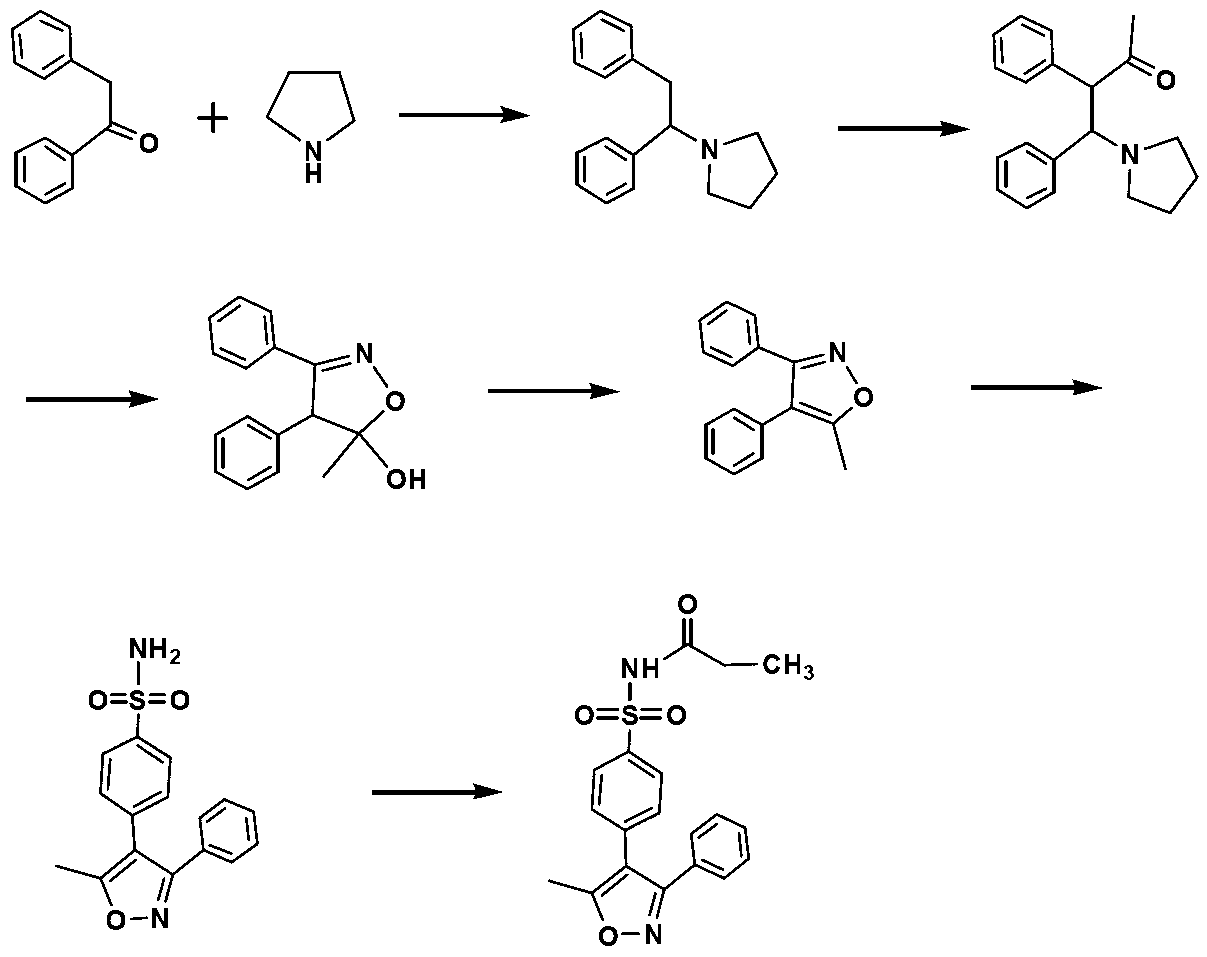
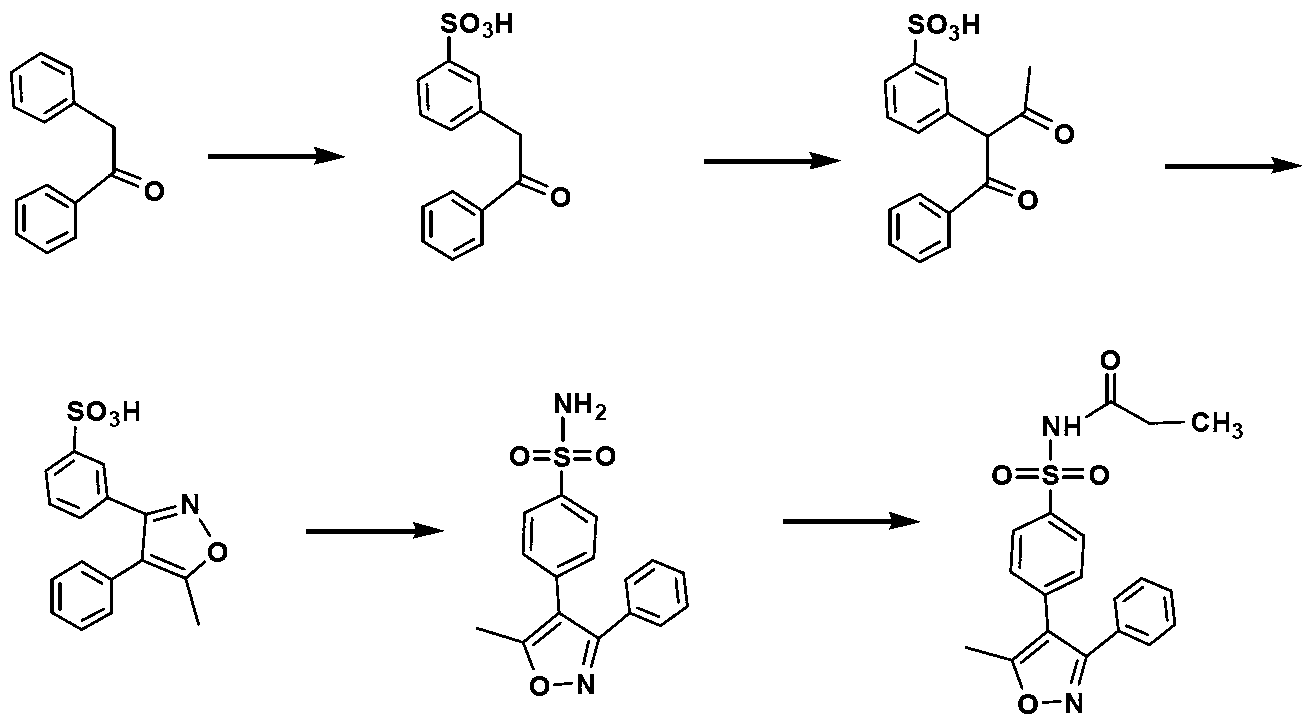
Parecoxib preparation method
A technology of parecoxib and valdecoxib, which is applied in the direction of organic chemistry, can solve the problems of expensive reagents, high equipment requirements, and long reaction time, and achieve simple and feasible process, low equipment requirements, and low raw material low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of Benzophenone Oxime
[0041] Add 200 g of 1,2-benzophenone (purchased from Tianjin Guangfu Fine Chemical Research Institute), 92.2 g of hydroxylamine hydrochloride (1.3 mol) (purchased from Tianjin Guangfu Fine Chemical Research Institute) and 400 ml of ethanol into the reaction bottle, and add 134 g of Ethylamine (1.3mol) (purchased from Tianjin Guangfu Fine Chemical Research Institute), heated up to 70-75°C and kept stirring for 4 hours, then cooled to 50-60°C, distilled under reduced pressure to remove ethanol to obtain an oily substance, added 500ml of water, oily After solidification, stir vigorously for 30 minutes to disperse, filter, wash with water (300ml×1), and wash with solvent (water:ethanol=2:1, 300ml×2). Dry at 50°C for 4-6 hours to obtain 194 g of white solid, yield 90%.
[0042] 2. Preparation of 4,5-dihydro-5-methyl-5-hydroxy-3,4-diphenylisoxazole
[0043] Under the protection of nitrogen, add 120g of benzophenone oxime and 500ml of t...
Embodiment 2
[0050] The preparation method of Example 2 is similar to that of Example 1, except that there is no preparation step of benzophenone oxime, and the commercially available benzophenone oxime (purchased from Tianjin Madsen Pharmaceutical Technology Co., Ltd.) is directly used for subsequent preparation. Valdecoxib as a white powder solid and parecoxib as a white solid were also obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com