Mass control method for clopidogrel splitting agent
A quality control method and a technology for splitting reagents, which are applied in the directions of measuring devices, material separation, and analysis materials, etc., can solve the problems of short service life of amino-bonded silica gel, high detection cost, and high price of sodium octane sulfonate. Long-term storage, high sensitivity, and high solution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The chromatographic conditions are:
[0034]
[0035] Preparation of sample solution: Accurately weigh 0.05g of solid sample, place it in a 100mL volumetric flask, add water to dissolve and dilute to the mark with water, acetonitrile or mobile phase, shake well, and the concentration of the obtained sample solution is 0.5mg / mL. Prepare 2 copies in parallel.
[0036]Preparation of reference solution: Accurately weigh different quality sodium camphorsulfonate reference substances, place them in 100mL volumetric flasks, add water to dissolve and dilute to the mark, and shake well. Prepare 2 copies in parallel.
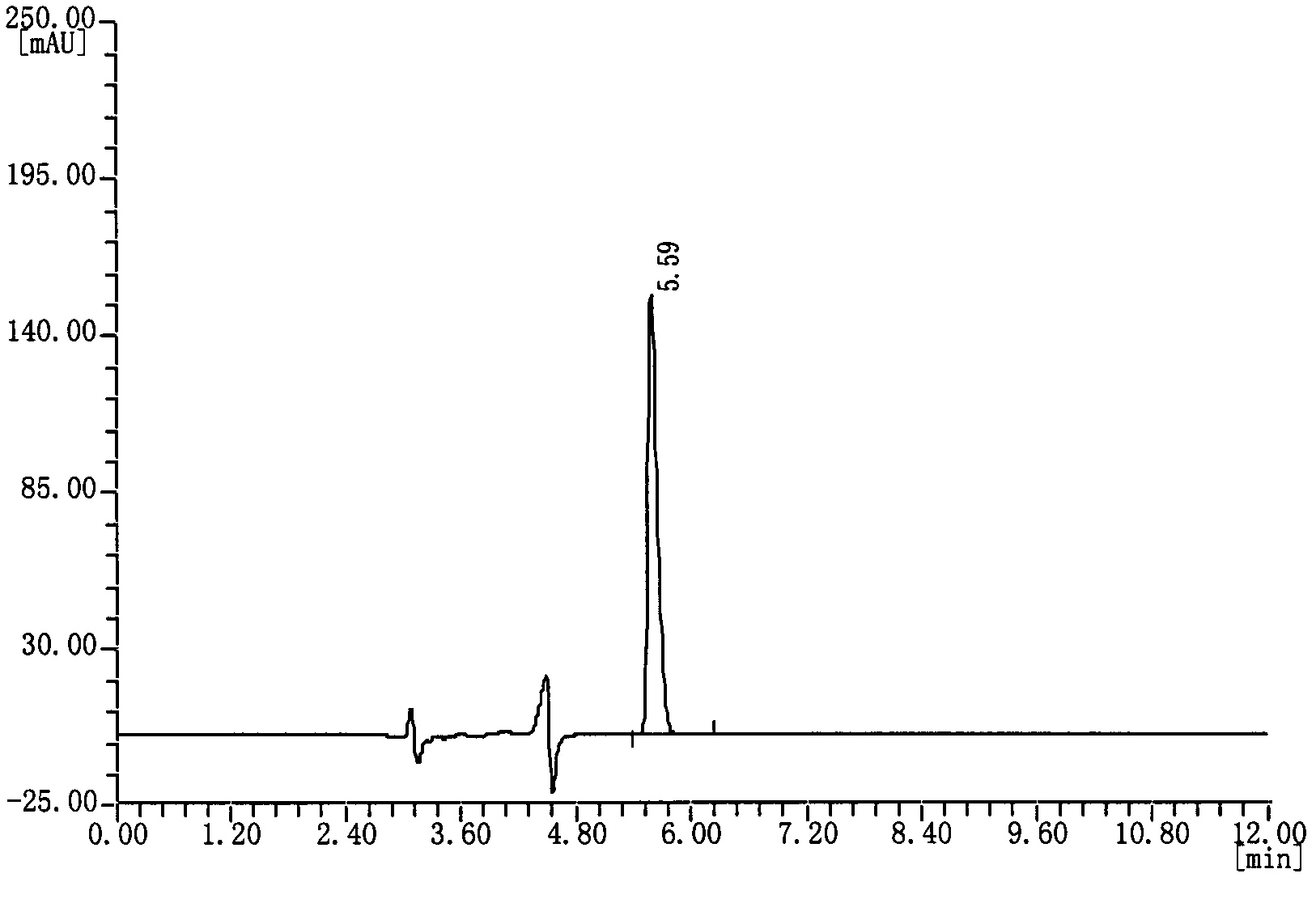
[0037] Measuring method: draw reference substance solution and need testing solution appropriate amount respectively, inject high-performance liquid chromatograph, record and obtain as follows: figure 1 The chromatogram shown. Each solution was repeated twice, and the content of camphorsulfonic acid in the test sample was calculated by the peak area using the...
Embodiment 2
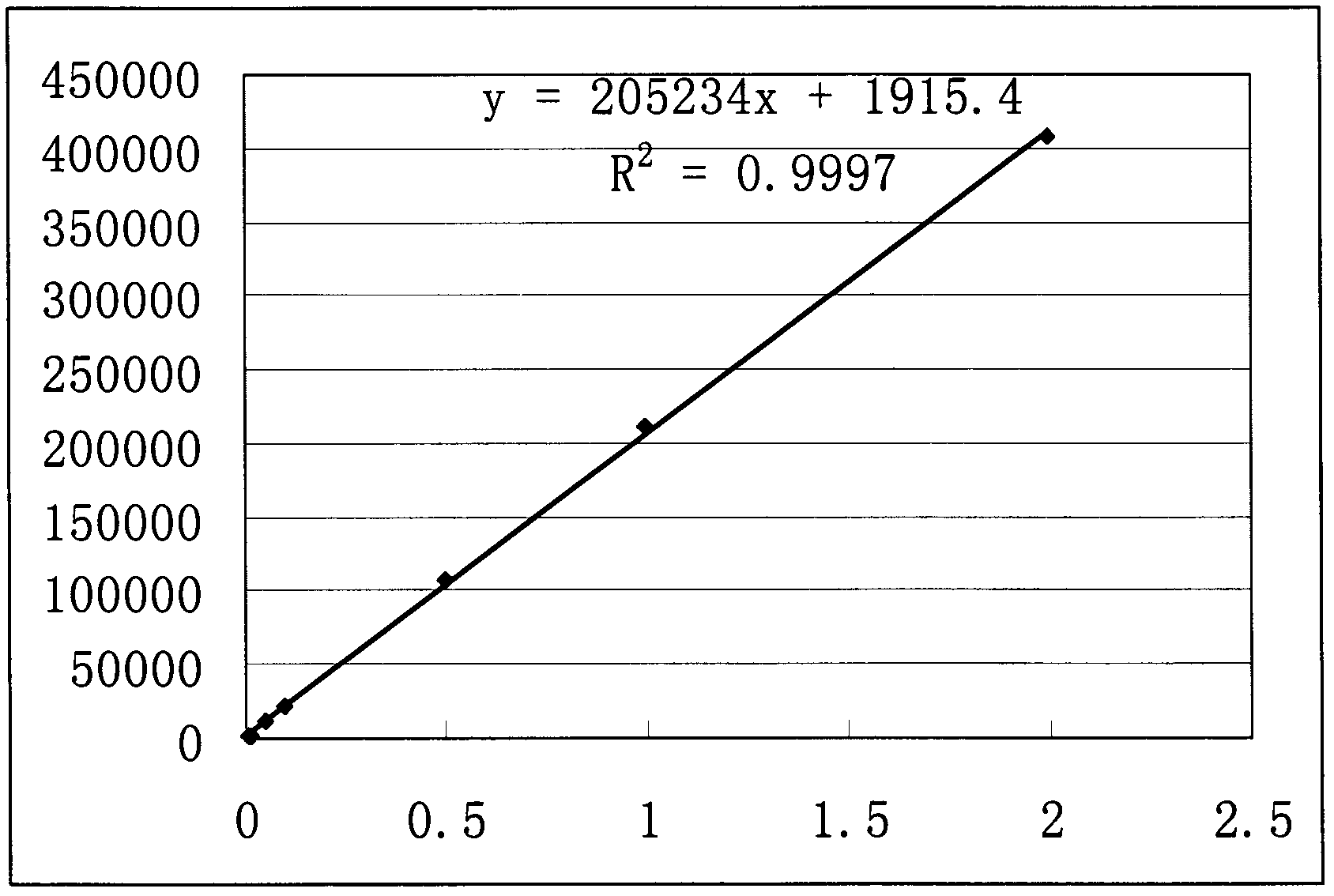
[0040] According to the method of Example 1, a 4.994 mg / mL sample solution was prepared. Dilute the sample solution with water in different proportions to make a solution with a concentration of 2, 1, 0.5, 0.1, 0.05, 0.01, and 0.005 mg / mL, and use the same chromatographic conditions as in Example 1 to measure from low concentration to high concentration. , each concentration was repeated 6 times, and the data shown in Table 1 were obtained. Taking the concentration (C) of camphorsulfonic acid as abscissa, taking the mean value (A) of the peak area of camphorsulfonic acid as ordinate drawing to obtain such as figure 2 Standard curve shown. The results showed that the linear relationship of camphorsulfonic acid was good in the concentration range of 0.005-2.0 mg / mL. The regression equation is: y=205234x+1915.4, r=0.9998.
[0041] Table 1 camphorsulfonic acid linear range experimental data
[0042]
Embodiment 3
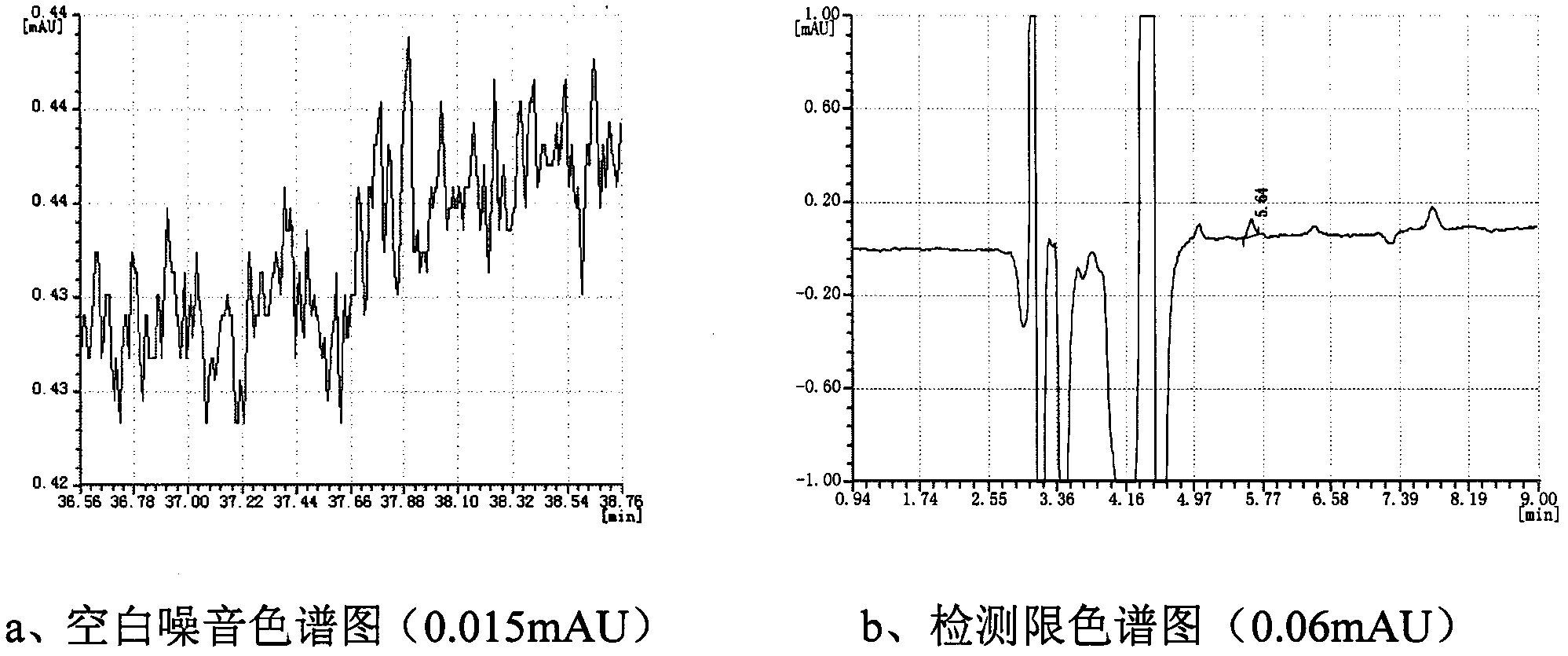
[0044] Prepare a 0.5 mg / mL sample solution according to the method in Example 1. Using the same chromatographic conditions as in Example 1, the determination was repeated 6 times to obtain the experimental data shown in Table 2. It can be seen from the results that the RSD value of the retention time of the main peak is 0.04%, and the RSD value of the peak area is 0.19%, indicating that the precision is good.
[0045] Table 2 camphorsulfonic acid precision experiment data
[0046] serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com