Aqueous suspension injection of cephalosporins and preparation method thereof
A technology of suspension injection and cephalosporins, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc. It can solve the problems of strong tissue irritation, short expiration date and poor stability of finished products, and achieve muscle stimulation Small effect, good redispersibility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 cephalosporin aqueous suspension of the present invention and preparation method thereof
[0036] Sample 1, 5% w / v cephalexin aqueous suspension and preparation method thereof
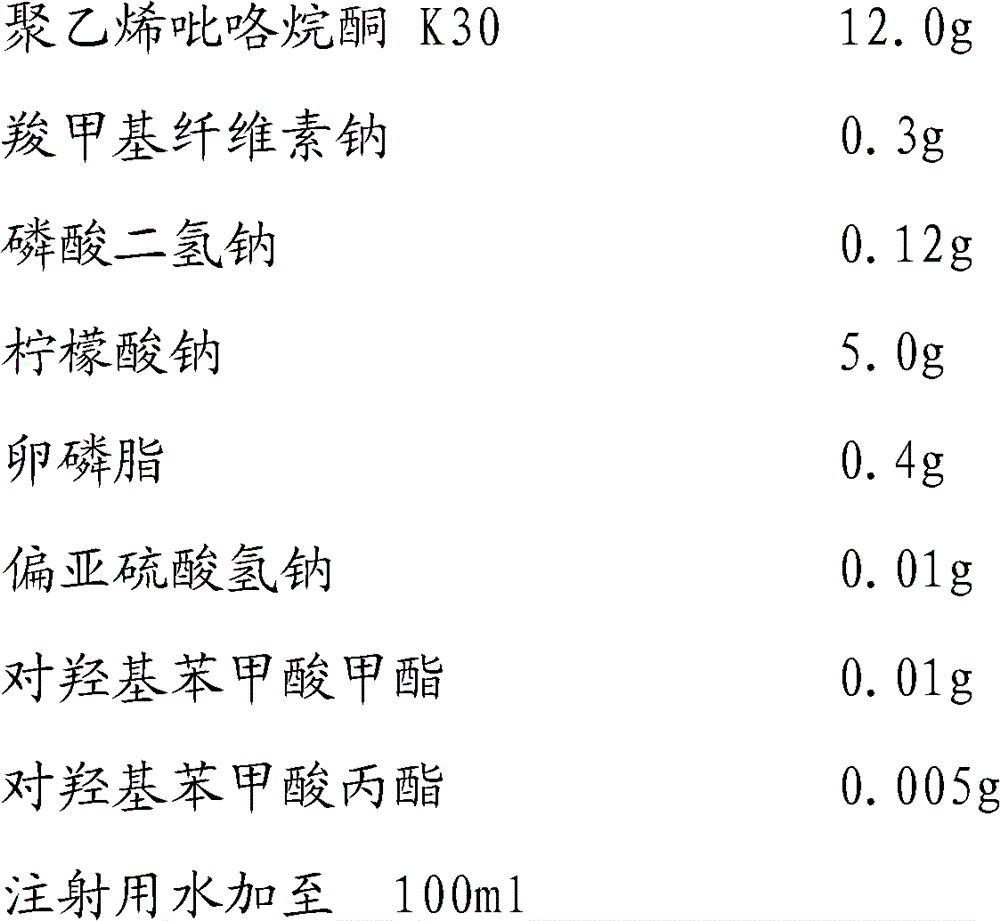
[0037]
[0038] Preparation steps:
[0039] 1) Weigh 1.0 g of sodium carboxymethyl cellulose, add it to 80 ml of water, heat slowly to about 60 ° C, stir while heating until the sodium carboxymethyl cellulose is completely dissolved, and set the volume to 100 ml to obtain 0.5% carboxymethyl cellulose Sodium cellulose solution.
[0040] 2) Weigh 10.0g of PVPK30, add a small amount of water to make a solution, add carboxymethylcellulose sodium solution 30ml, sorbitol 5.0g, sodium citrate 3.0g, poloxamer 0.5g, dihydrogen phosphate Add 0.1g of potassium, 0.01g of sodium formaldehyde sulfoxylate, 0.01g of methyl p-hydroxybenzoate, and 0.005g of methyl p-hydroxybenzoate into 50ml of water, stir to dissolve completely.
[0041] 3) Add 5.0 g of micronized cephalexin (Shijiazhuang Taih...
Embodiment 2
[0070] Embodiment 2, the appearance inspection that contains cephalosporin aqueous suspension sample
[0071] Take the aqueous suspension samples 1, 2, 3, and 4 of the present invention, observe their appearance properties, wall hanging phenomenon, measure their sedimentation volume ratio and viscosity, and evaluate the appearance quality.
[0072] For the determination method of sedimentation volume ratio and content, please refer to the second volume of "The Veterinary Pharmacopoeia of the People's Republic of China" in 2010.
[0073] Table 2, the quality inspection of the suspension sample prepared by the inventive method
[0074]
[0075] It can be seen from the table above that the appearance of aqueous suspension injection samples 1, 2, 3, and 4 is milky white liquid, and there is no wall hanging phenomenon after shaking. 99-101% of the marked amount meets the appearance quality requirements of the suspension injection.
Embodiment 3
[0076] Embodiment 3, viscosity and needle-drawing inspection
[0077] Take samples 1, 2, 3, and 4 of the aqueous suspension injection prepared by the present invention, and compare them with oily injections currently on the market, and compare the differences in viscosity and needle withdrawal.
[0078] Control 1: 5% w / v ceftiofur hydrochloride oily injection (Shu Kejian, produced by Shandong Qilu Animal Health Products Co., Ltd., batch number: 100801)
[0079] Control 2: 5% w / v ceftiofur hydrochloride oily injection (Sujieling, Upjohn Company, USA, batch number: 0A3H9,)
[0080] Viscosity measurement method: Take an appropriate amount of the test product, put it in a water bath in the container and keep the temperature at 20°C. Use a 5ml graduated straw (the diameter of the inner wall at the outlet end of the straw is 2mm) to draw 5ml of the test product, let it flow out naturally, and record the time required to flow out 5ml.
[0081] Needle-drawing property: Take an appro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com