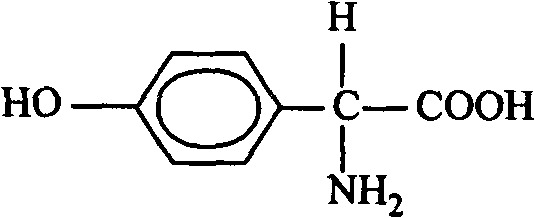
Novel synthesis process of DL-p-hydroxyphenylglycine
A p-hydroxyphenylglycine, a new process technology, applied in the field of compound preparation, can solve the problems of long reaction time, low yield, difficult treatment of ammonia nitrogen wastewater, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0011] 1. Put 240Kg into a 5000L enamel reaction kettle, 30Kg of dodecyl dimethyl benzyl ammonium chloride, 1000Kg of 29% glyoxylic acid, 600Kg of 4-nitrophthalimide, 300Kg of phenol, and stir rapidly Evenly, stir and react at 65±2°C for 10 hours, then cool to 25°C, filter to obtain about 600Kg of 4-nitrophthalic acid solid, and the recovery rate is ≥90%.
[0012] 2. Cool the above filtrate in ice water for 1 hour, filter with suction, and wash the filter cake with ice water and ethanol in turn until it is colorless. Dry at 70-80°C to obtain about 395Kg of a milky white solid, with a yield of ≥74%.
[0013] 3. Recycling of glyoxylic acid and phase transfer catalyst: the above mother liquor is distilled under reduced pressure and concentrated to one-third of the original volume, about 400Kg. The main components are glyoxylic acid and phase transfer catalyst, which can directly replace part of glyoxylic acid and A phase transfer catalyst was used in the synthesis of DL-p-hydrox...
example 2
[0015] 1. In a 5000L enamel reaction kettle, put 400Kg of recovered mother liquor of glyoxylic acid and phase transfer catalyst, 5Kg of dodecyl dimethyl benzyl ammonium chloride, 800Kg of 29% glyoxylic acid, 4-nitrophthalamide Imide 600Kg, phenol 240Kg, stir quickly evenly, stir and react at 65±2°C for 10h, then cool to 25°C, filter to obtain about 600Kg of 4-nitrophthalic acid solid, recovery rate ≥ 90%.
[0016] 2. Cool the above filtrate in ice water for 1 hour, filter with suction, and wash the filter cake with ice water and ethanol in turn until it is colorless. Dry at 70-80°C to obtain about 380Kg of a milky white solid, with a yield of ≥71%.
[0017] 3. Recovery and application of glyoxylic acid and phase transfer catalyst: the above mother liquor is applied mechanically according to the method in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com