High-efficiency expression method of human interleukin-10 (hIL-10)
A technology for expressing vectors and exogenous proteins, applied in the field of bioengineering, can solve the problems of poor results, difficult to achieve, low high-copy strains, etc., achieve good stability, avoid endoplasmic reticulum pressure, and prevent high-efficiency expression clones. The effect of losing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the construction of Pichia pastoris high-efficiency expression vector pUCZ9K, the specific process includes the following steps:
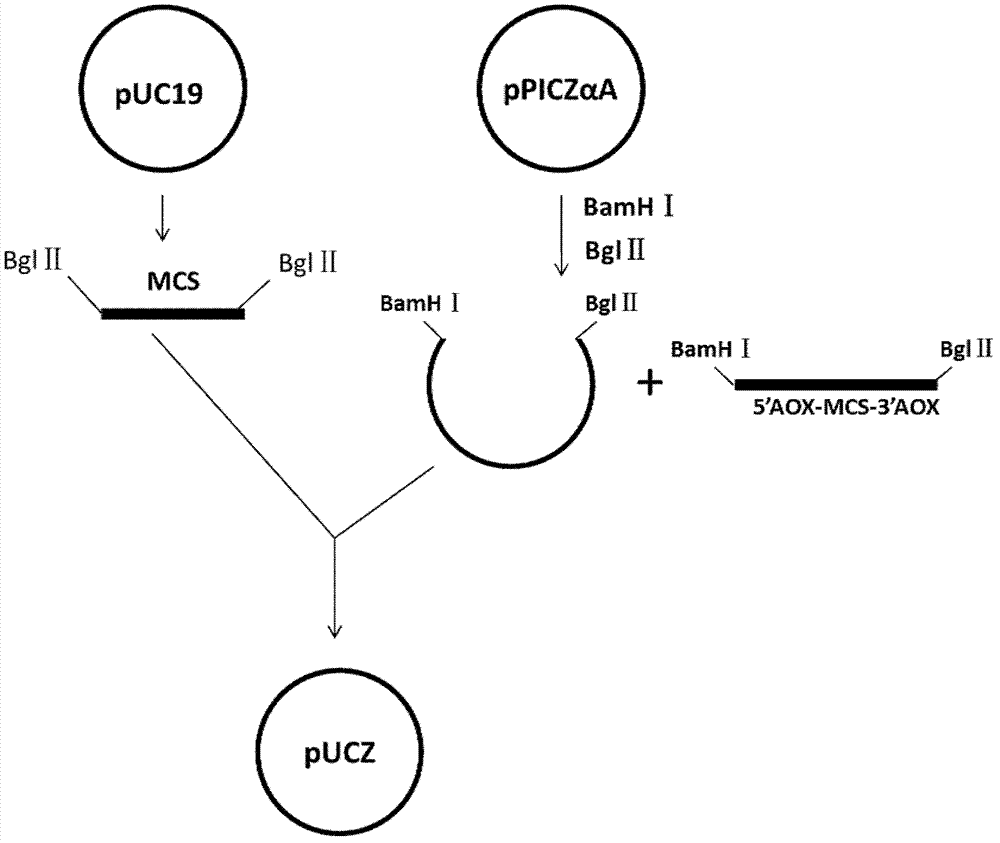
[0046] 1.1. Construction of pUCZ plasmid
[0047] The multiple cloning site (MCS) was amplified by PCR using the pUC19 plasmid (purchased from Bao Bioengineering) as a template, and one BglII site was introduced at each of its two ends; the pPICZαA plasmid was digested with BamHI and BglII to recover TEF1- The EM7-Zeocin-CYC1TT-pUC ori fragment was ligated with the recovered MCS fragment after digestion with BglII, and the ligated product was transformed into competent Escherichia coli DH5α (purchased from Treasure Bioengineering), and positive recombinants were screened. The positive recombinant plasmid was sequenced by Yingjun Company. The results showed that the MCS fragment was correctly connected with the TEF1-EM7-Zeocin-CYC1TT-pUCori sequence and the sequence was correct, and it was named pUCZ. The construction process is...
Embodiment 2
[0143] Example 2, Construction of IL-10-His / pUCZ9K Expression Vector and Screening of Yeast High Expression Strain
[0144] 2.1. Construction of hIL-10-His / pUCZ9K recombinant expression plasmid
[0145] 2.1.1. Amplification of hIL-10 gene
[0146] TRIzol (purchased from Invitrogen Company, Cat. No. 15596-018) was used to extract the total RNA of mononuclear cells from isolated healthy human peripheral blood (purchased from Hefei Blood Bank) according to its instructions, and the total RNA was extracted in Turbo Reverse Transcriptase (purchased from Beijing Yuanpinghao Co., Ltd.). , Cat. No. PC108), oligo dT (purchased from Shanghai Sangong, Cat. No. B0181) was used as a primer to synthesize human cDNA, using the synthesized cDNA as a template, the forward primer 5'-ATGCACAGCTCAGCACTGCTCTG-3' and the reverse primer 5 PCR was performed on '-GTTTCGTATCTTCATTGTCATG-3', and the PCR product was inserted into the TA vector pMD18-T (purchased from Treasure Bioengineering). The plasmi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com