High dispersion supported nano noble metal catalyst and preparation method and application thereof
A precious metal catalyst and a supported technology, which are applied in the field of supported nano precious metal catalysts and their preparation, can solve the problems of cumbersome process, large particle size of active components in the catalyst, poor dispersibility and stability, etc., so as to improve the structural stability, Excellent catalytic performance, mild reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
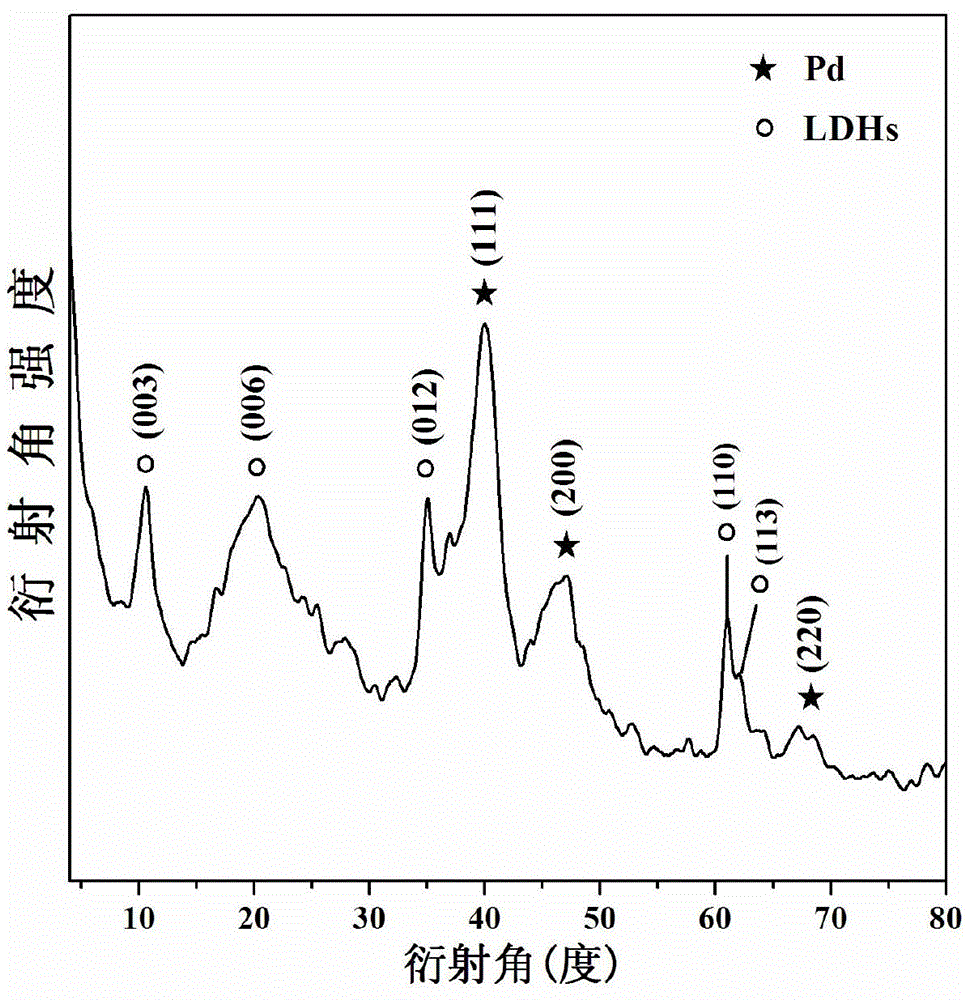
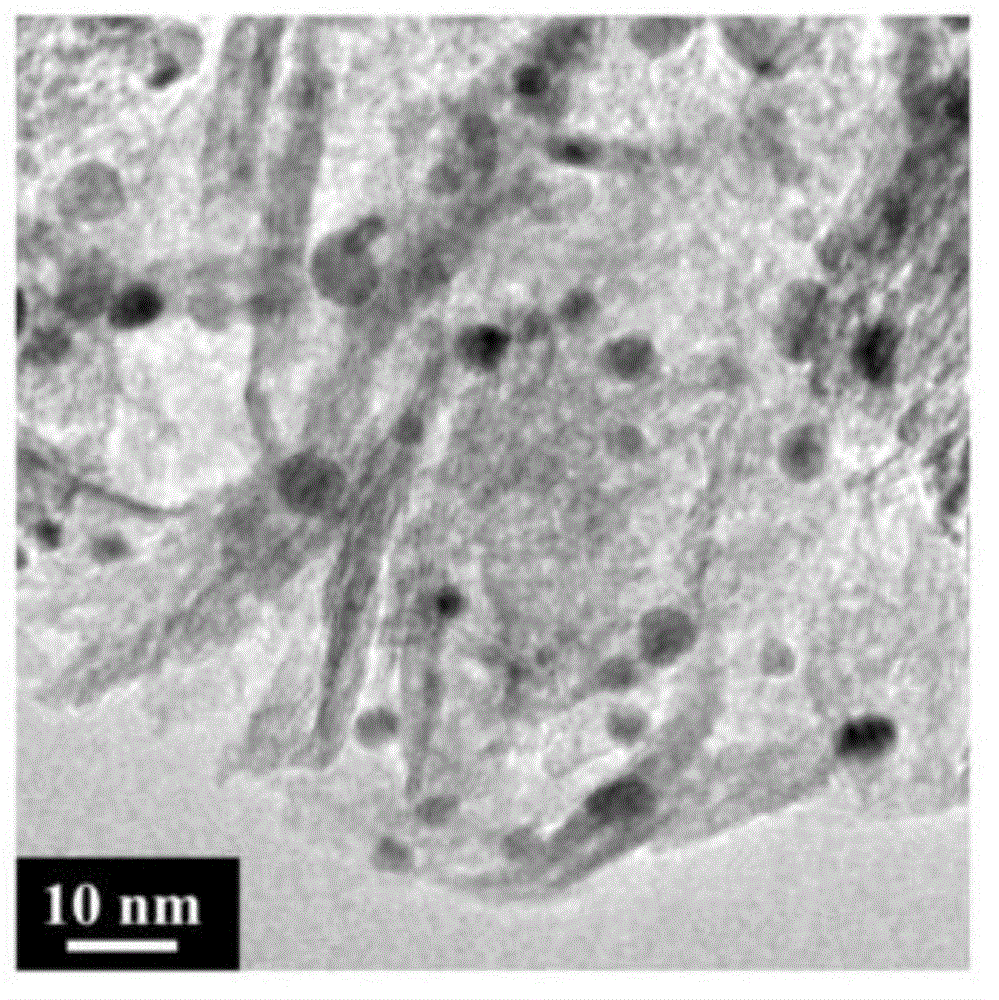
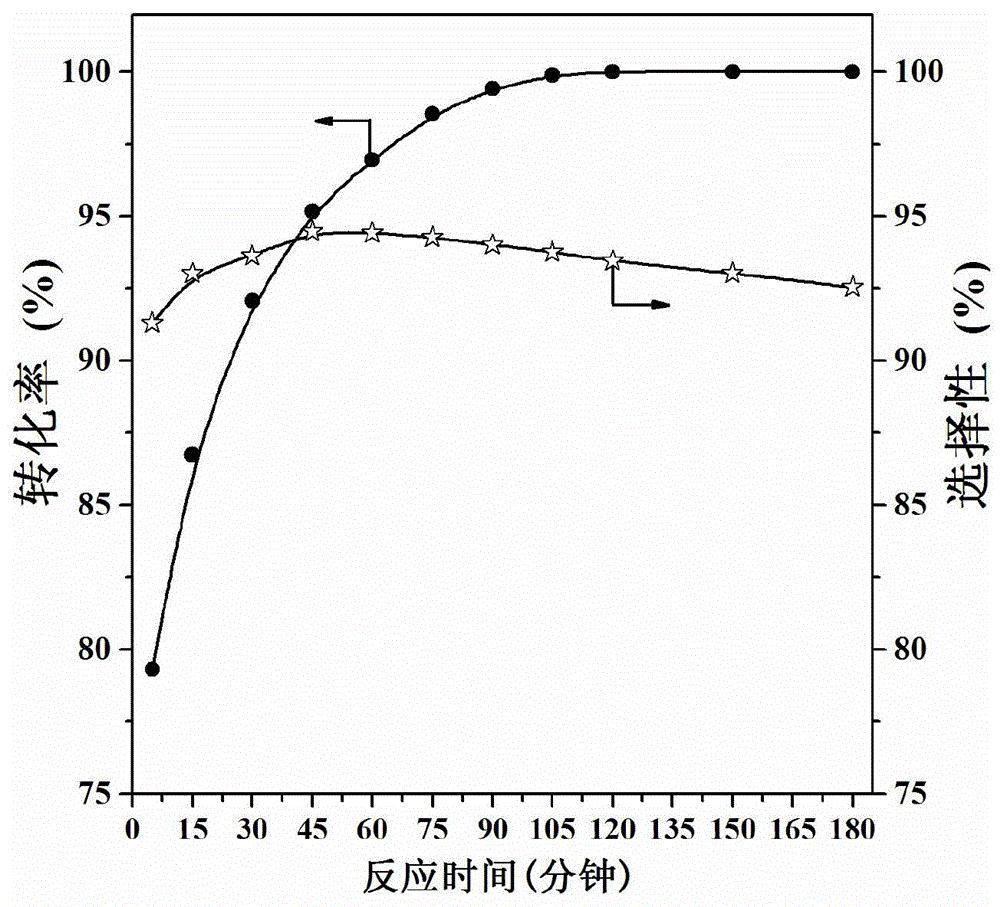
Image
Examples
Embodiment 1
[0019] A. Add 1.5384g of Mg(NO 3 ) 2 ·6H 2 O and 1.1254g of Al(NO 3 ) 3 9H 2 O is prepared into 50ml mixed salt solution; take 0.04mol / L H with a pipette 2 PdCl 4 Add 5ml of the solution to the above mixed solution;
[0020] B. Add the mixed salt solution of step A into the reaction container, ultrasonically disperse it for 20 minutes, and mix it evenly; add LiOH solution with a concentration of 0.18mol / L to the above mixed metal salt solution, and adjust the pH of the solution to 8.0; in N 2 atmosphere, crystallize at a reaction temperature of 40°C for 8 hours; cool to room temperature, and centrifuge and wash with deionized water until neutral to obtain a precipitate.
[0021] C. Take by weighing 4.4588g glucose and dissolve in 50ml aqueous ethanol (V C2H5OH / V H2O =1:3), slowly pour the precipitate obtained above into the aqueous glucose alcohol solution, and ultrasonically disperse it for 20 minutes; pour the mixed solution into the reaction kettle, seal it, put ...
Embodiment 2
[0024] 2.3076g of Mg(NO 3 ) 2 ·6H 2 O and 1.1254g of Al(NO 3 ) 3 9H 2O is prepared into a 50ml mixed solution; use a pipette to take 0.04mol / L of H 2 PdCl 4 Add 5ml of the solution to the above mixed solution; weigh 0.648g of LiOH solid and dissolve it in 150ml of deionized water to prepare an alkaline solution, wherein the concentration of the LiOH alkaline solution is 0.18mol / L.
[0025] Add the prepared mixed metal salt solution into the reaction vessel, ultrasonically disperse it for 20 minutes to make it evenly mixed; add the alkaline solution dropwise into the above mixed metal salt solution with a burette, and adjust the pH of the solution to 7.0; 2 atmosphere, crystallize at a reaction temperature of 40°C for 8 hours; cool to room temperature, and centrifuge and wash with deionized water until neutral to obtain a precipitate.
[0026] Take by weighing 2.6753g glucose and dissolve in 50ml aqueous methanol (V CH3OH / V H2O =1:1), slowly pour the precipitate obtai...
Embodiment 3
[0029] 1.9230g of Mg(NO 3 ) 2 ·6H 2 O and 1.1254g of Al(NO 3 ) 3 9H 2 O is prepared into a 50ml mixed solution; use a pipette to take 0.04mol / L of H 2 PtCl 4 Add 5ml of the solution to the above mixed solution; weigh 0.648g of LiOH solid and dissolve it in 150ml of deionized water to prepare an alkaline solution, wherein the concentration of LiOH is 0.18mol / L.
[0030] Add the prepared mixed metal salt solution into the reaction vessel, ultrasonically disperse for 20 minutes to make it evenly mixed; slowly add the alkaline solution dropwise into the above mixed metal salt solution, and adjust the pH of the solution to 6.5; 2 Atmosphere, crystallize at a reaction temperature of 30°C for 8 hours; cool to room temperature, and centrifuge and wash with deionized water until neutral to obtain a precipitate.
[0031] Take by weighing 1.7835g glucose and dissolve in 60ml aqueous ethanol (V C2H5OH / V H2O =1:2), slowly pour the precipitate obtained above into the aqueous gluco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com