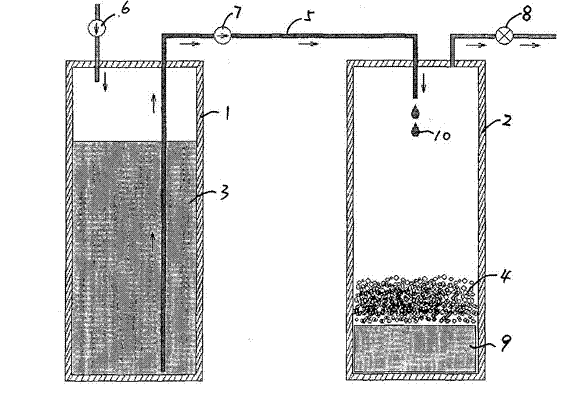
Portable hydrogen generator
A portable and generator technology, applied in the field of portable hydrogen generators, can solve the problems of slow hydrolysis and self-release of hydrogen, potential safety hazards, and difficulty in miniaturization and safety of hydrogen-producing equipment, so as to omit safety precautions and achieve a complete and complete reaction. , Eliminate the effect of product agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: with 1.0g NaBH 4 According to the B atom molar ratio of 1:1 and H 3 BO 3 Mix, add 100 microliters of water each time at normal temperature and pressure until 1500 microliters of water is added, a total of about 1.2 liters of hydrogen is generated, and the reaction is complete. Each reaction was completed within 3-7 seconds after each addition of 100 microliters of water. The results show that the reaction process can be controlled by controlling the addition of water.
[0029] The same reaction starting material, when all 1.5ml of water is added at one time, the reaction ends within 10 seconds, and the measured maximum instantaneous hydrogen desorption rate> 3 liters / second.
[0030] When the NaBH 4 and H 3 BO 3 After ball milling to about 100 microns and mixing, the reaction speed is faster after adding water. After adding 100 microliters of water each time, the reaction is completed within 3 seconds, and the average hydrogen production rate is 33.3...
Embodiment 2
[0033] Embodiment 2: 1.0g NaBH 4 With metaboric acid (boric acid through 120 o C dehydration treatment) mixture (the molar ratio of B atoms is 1:1), after adding 1.5g of water at normal temperature and pressure, the reaction process is similar to that of Example 1, with no significant difference. The reaction product is also Na 2 B 4 o 7 ·5H 2 0. The reaction equation is as follows:
[0034] .
Embodiment 3
[0035] Embodiment 3: the NaBH 4 Mix with metaboric acid (or boric acid) powder according to the B atom molar ratio of 1:0.7, add water at normal temperature and pressure, the experimental phenomenon is similar to Example 1, and the reaction speed has no obvious change.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com