Method for artificially synthesizing gem-class malachite
A technology of artificial synthesis and malachite, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., can solve the problems that cannot be popularized and popularized, and achieve the effects of easy control of the preparation process, simple process, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
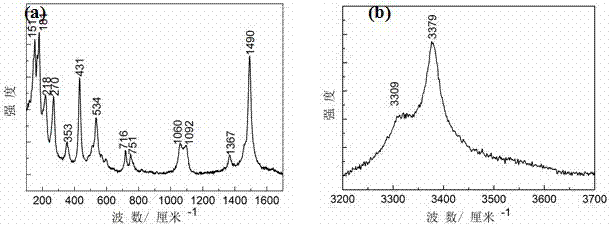
[0041] Weigh 42g NaHCO 3 , Add 500 ml of deionized water to dissolve it and place it in a beaker as a salt solution for evaporation; put the polished copper-tin alloy suspended on the surface of the salt solution and seal the beaker; use a water bath to keep the beaker constant Keep warm, control temperature at 90 °C, keep constant temperature for 100 days, use the same concentration of NaHCO every day 3 The solution renews the salt solution in the beaker. In this example, the NaHCO used to renew the solution in the beaker 3 The solution is made up of 42g NaHCO 3 NaHCO with 500 ml deionized water 3 Solution; after the end, the sample should be removed and ultrasonically cleaned.
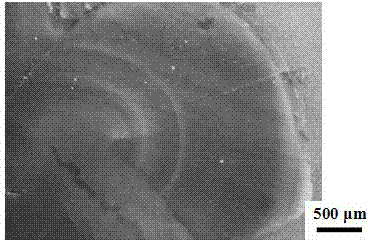
[0042] figure 1 An optical microscope photo of the malachite prepared in this example, it can be seen in the figure that the prepared malachite is opaque and dark green, with a striped pattern of shades similar to natural malachite, with a particle size of 5-20 mm , To meet the basic requirements as a ge...
Embodiment 2
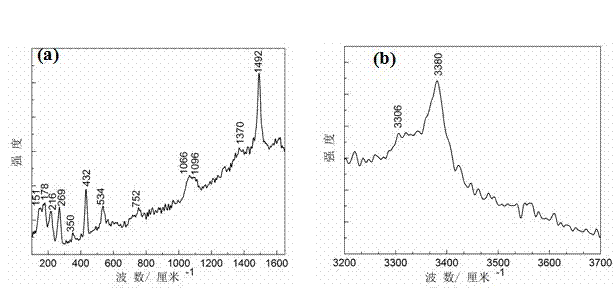
[0044] Weigh 42g NaHCO 3 , Add 500 ml of deionized water to dissolve it and place it in a beaker as a salt solution for evaporation; put the polished copper-tin alloy suspended on the surface of the salt solution and seal the beaker; use a water bath to keep the beaker constant Keep warm and control the temperature at 90 °C; in the first stage of the reaction, that is, the first to 10 days, the same concentration of NaHCO is used every day 3 The solution renews the salt solution in the beaker. In this example, the NaHCO used to renew the solution in the beaker 3 The solution is made up of 42g NaHCO 3 NaHCO with 500 ml deionized water 3 Solution; In the second stage, the 11th-15th day, the same as the first stage, the same initial concentration of NaHCO is still used every day 3 The solution renews the salt solution in the beaker, but at the same time smear CuCl on the surface of the sample 2 Powder, smear 0.02 g per square centimeter on average, keep other reaction conditions unch...
Embodiment 3
[0047] Weigh 42g NaHCO 3 , Add 500 ml of deionized water to dissolve it and place it in a beaker as a salt solution for evaporation; place the polished metal copper suspended on the surface of the salt solution and seal the beaker; use a water bath to keep the beaker at constant temperature , Control the temperature to 90 °C, and use the same concentration of NaHCO every day in the first stage of the reaction, that is, the first to 10 days 3 The solution renews the salt solution in the beaker. In this example, the NaHCO used to renew the solution in the beaker 3 The solution is made up of 42g NaHCO 3 NaHCO with 500 ml deionized water 3 Solution; In the second stage, the 11th-15th day, the same as the first stage, the same initial concentration of NaHCO is still used every day 3 The solution renews the salt solution in the beaker, but at the same time smear CuCl on the surface of the sample 2 Powder, smear 0.05g per square centimeter on average, keep other reaction conditions uncha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com