Amphipathic polysaccharide/polypeptide block polymer containing azobenzene group and preparation method and application of block polymer
An amphiphilic polysaccharide and block polymer technology, which is applied in the field of amphiphilic polysaccharide/polypeptide block polymer and its preparation, can solve problems such as no research reports containing azobenzene, and achieve excellent biocompatibility The effect of high stability and biodegradability, excellent performance and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
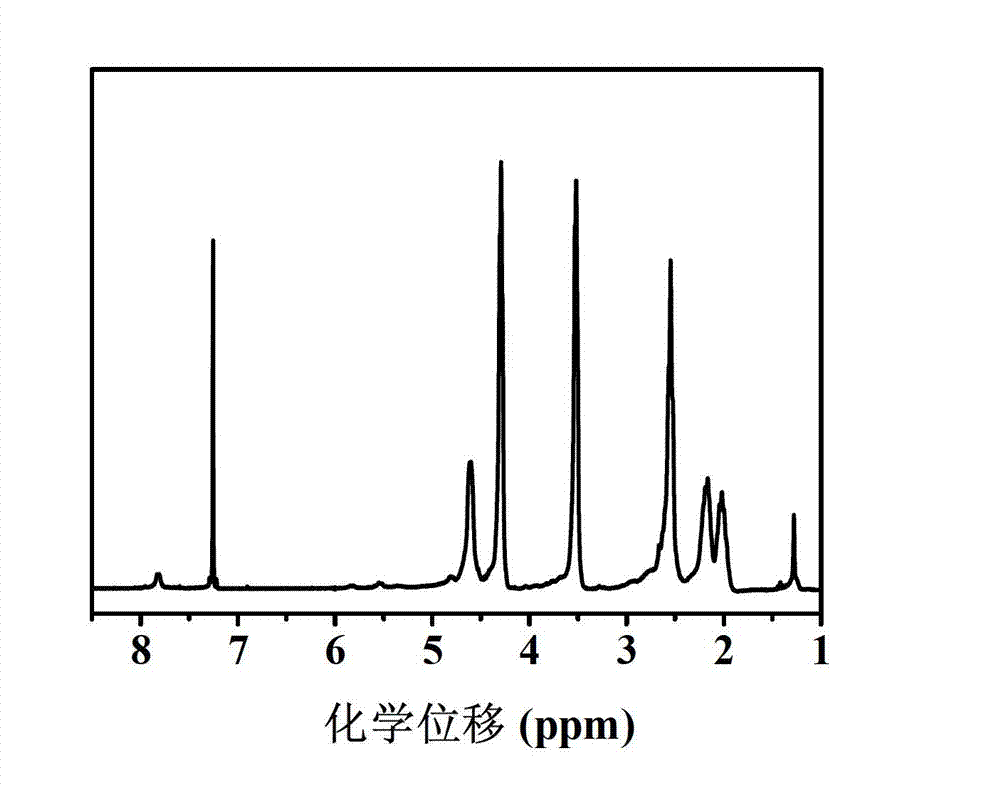
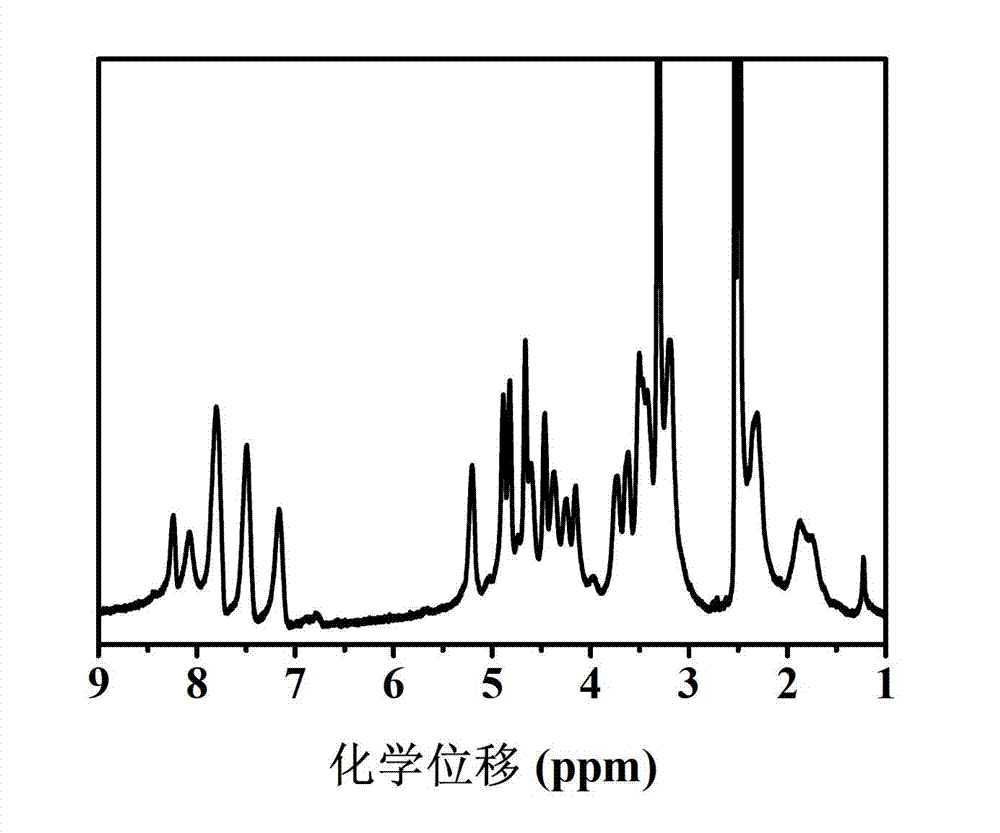
[0055] (1) Under the protection of argon, add 10.40g of γ-2-chloroethyl-L-glutamate and 3.60g of trimeric phosgene into a three-necked round-bottomed flask containing 100mL of tetrahydrofuran, react at 50°C for 1 hour, and then depressurize at 30°C THF was distilled off to obtain an oily substance; 150 mL of ethyl acetate was added to the obtained oily substance, shaken up to obtain a mixed solution of ethyl acetate; the mixed solution of ethyl acetate was washed twice with 120 mL of cold 5wt% sodium bicarbonate solution, and transferred to Put it into a pear-shaped separatory funnel to separate the organic phase, dry it overnight with 15g of anhydrous magnesium sulfate, filter it, and evaporate the filtrate to dryness at 30°C, the white needles obtained are γ-2-chloroethyl-L-glutamic acid Ester ring internal acid anhydride (CELG-NCA); put 0.0172g azidoethylamine and 2.36g CELG-NCA in a reaction flask containing 10mL of anhydrous N,N-dimethylformamide at a molar ratio of 1:50, ...
Embodiment 2
[0073](1) Under the protection of argon, add 5.60g of γ-2-chloroethyl-L-glutamate and 2.40g of trimerized phosgene into a three-neck round bottom flask containing 50mL of tetrahydrofuran, react at 50°C for 1h, and then decompress at 35°C The tetrahydrofuran was distilled off to obtain an oily substance; 100 mL of ethyl acetate was added to the obtained oily substance, and shaken to obtain a mixed solution of ethyl acetate; the mixed solution of ethyl acetate was washed twice with 85 mL of cold 5wt% sodium bicarbonate solution, and transferred to Put it into a pear-shaped separating funnel to separate the organic phase, dry it overnight with 10g of anhydrous magnesium sulfate, filter it, evaporate the filtrate to dryness at 35°C, and obtain the white needle-shaped product CELG-NCA; Amine and 2.36g CELG-NCA were placed in a reaction flask containing 12mL of anhydrous N,N-dimethylformamide, argon was introduced, and the reaction was carried out at room temperature for 3 days to ob...
Embodiment 3
[0090] (1) Under the protection of argon, add 2.80g of γ-2-chloroethyl-L-glutamate and 1.20g of trimerized phosgene into a three-necked round-bottomed flask containing 100mL of tetrahydrofuran, react at 60°C for 0.5h, and then 33°C Remove tetrahydrofuran by distillation under reduced pressure to obtain an oil; add 125 mL of ethyl acetate to the obtained oil and shake well to obtain a mixed solution of ethyl acetate; wash the mixed solution of ethyl acetate twice with 100 mL of cold 5wt% sodium bicarbonate solution , transferred to a pear-shaped separating funnel to separate the organic phase, dried overnight with 12.5g of anhydrous magnesium sulfate and filtered, and evaporated the filtrate at 30°C to obtain the white needle-shaped product CELG-NCA; 0.0044g Azideethylamine and 2.36g CELG-NCA were placed in a reaction flask containing 15mL of anhydrous N,N-dimethylformamide, argon gas was passed through, and the reaction solution was obtained at room temperature for 3 days; Pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
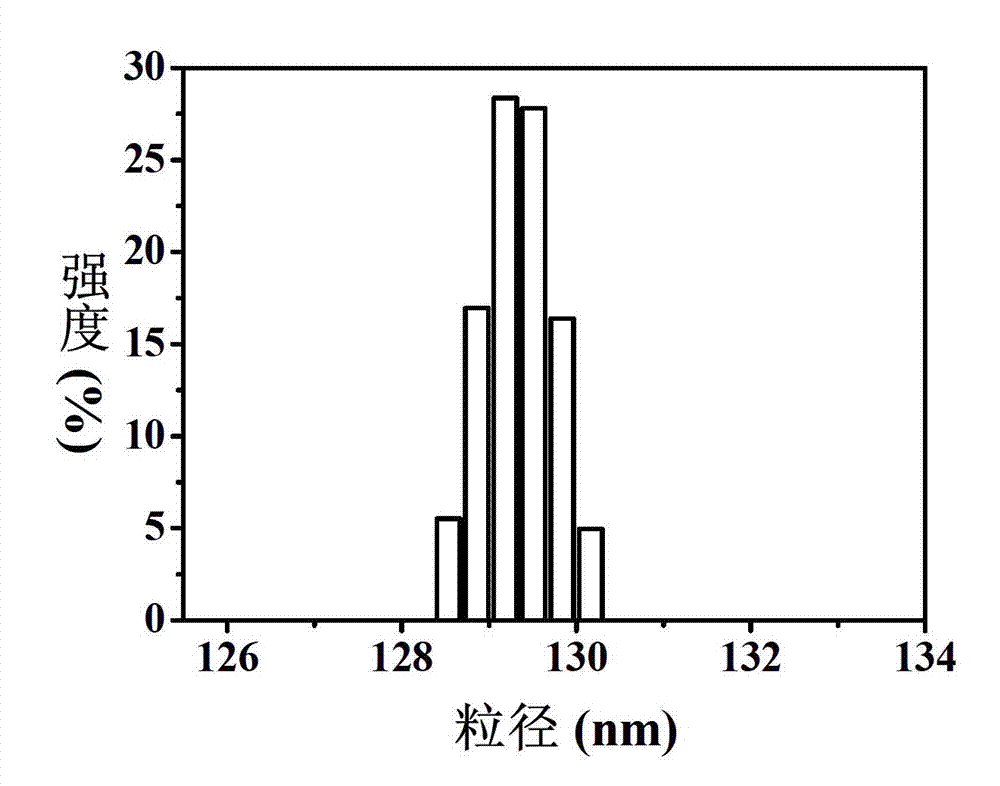
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com