Eye drop containing VEGF antagonist
An eye drop and antagonist technology, applied in the field of eye drops containing VEGF antagonist, can solve the problems of instability, limited effect, high cost and the like, and achieve the effects of saving production cost, accurate curative effect and extremely high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
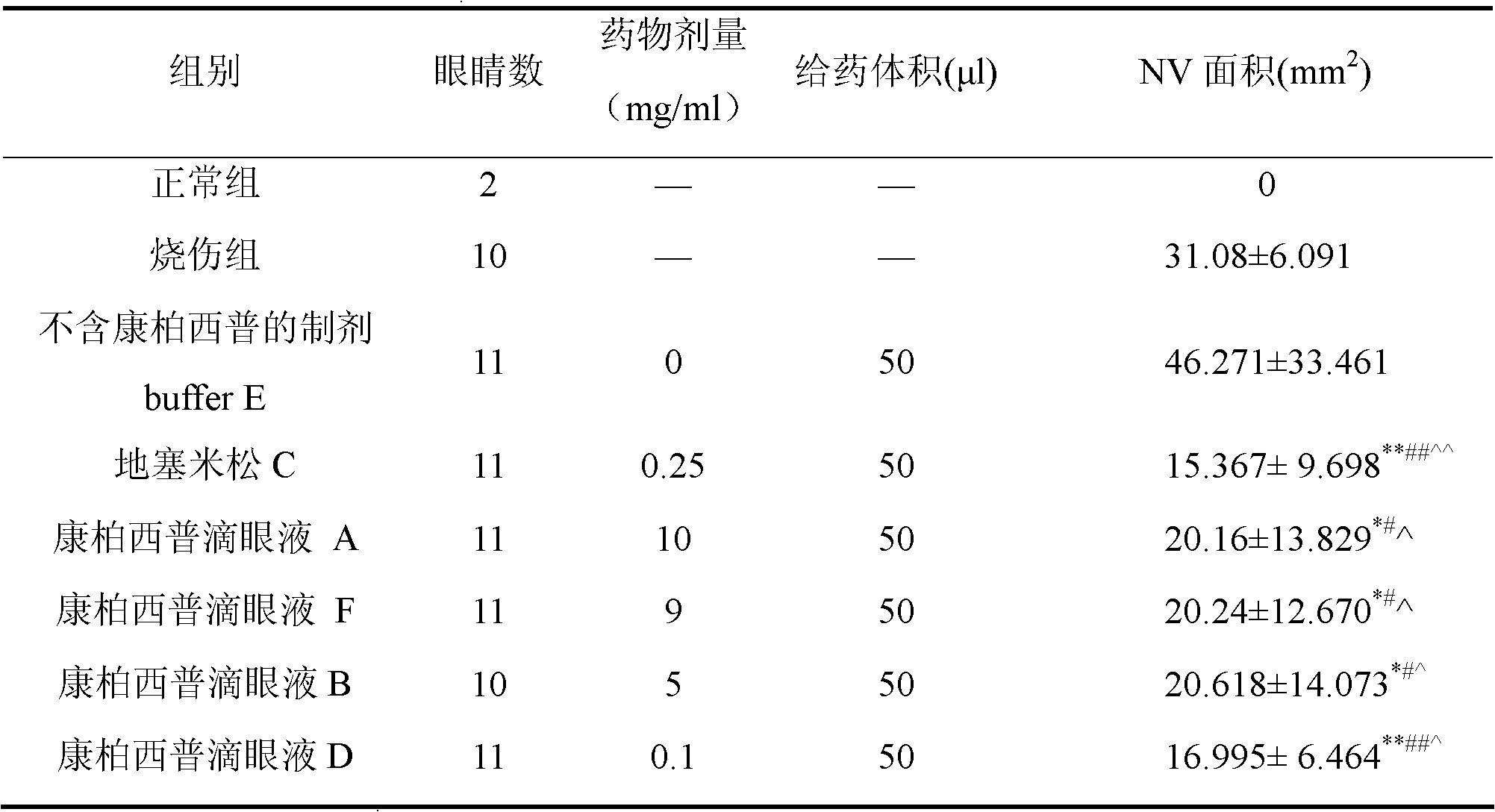
[0053] Example 1, Conbercept Eye Drops Study on Corneal Neovascularization Induced by Alkali Burn
[0054] Drugs: Chlortetracycline Hydrochloride Eye Ointment, specification batch number: 2.0g / bottle, 411002, valid until December 2014, Chongqing Kerui Pharmaceutical Co., Ltd.; lidocaine hydrochloride injection, specification batch number: 5ml / bottle, 0.1g / bottle, Valid until April 2012, Tianjin Pharmaceutical Jiaozuo Co., Ltd.
[0055] Reagent: sodium hydroxide (NaOH), specification batch number: 500g / bottle, 20091223, Chengdu Kelong Chemical Reagent Factory.
[0056] Samples tested:
[0057] A, Conbercept eye drops (prepared according to Example 3) 10mg / ml, colorless transparent liquid, 1ml / bottle, batch number: FR1108001, stored at 2-8°C, added dropwise to the surface of the eye when used;
[0058] B, Conbercept eye drops (prepared according to Example 3) 9mg / ml, colorless transparent liquid, 1ml / bottle, batch number: FR1108002, stored at 2-8°C, added dropwise to the surfa...
Embodiment 2
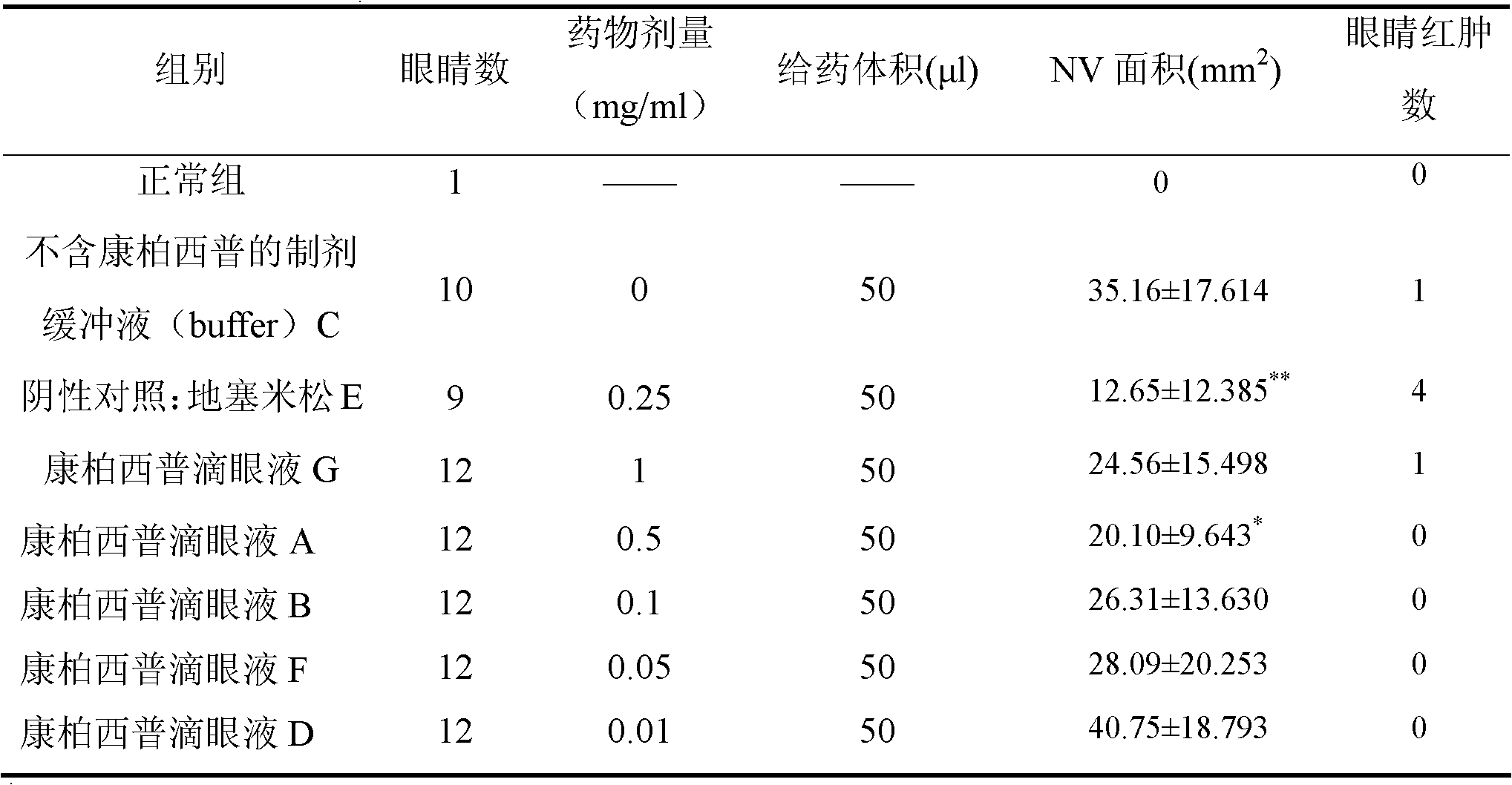
[0073] Example 2 Effect of low-dose Conbercept eye drops on corneal NV growth caused by alkali burn
[0074] Drugs: Chlortetracycline Hydrochloride Eye Ointment, specification batch number: 2.0g / bottle, 411002, valid until December 2014, Chongqing Kerui Pharmaceutical Co., Ltd.; lidocaine hydrochloride injection, specification batch number: 5ml / bottle, 0.1g / bottle, Valid until April 2012, Tianjin Pharmaceutical Jiaozuo Co., Ltd.
[0075] Reagent: sodium hydroxide (NaOH), specification batch number: 500g / bottle, 20091223, Chengdu Kelong Chemical Reagent Factory.
[0076] Samples tested:
[0077] A, Conbercept Eye Drops (prepared according to Example 3) 0.5mg / ml, colorless transparent liquid, 800 μl / bottle, batch number: 20111001, stored at 2-8°C, added dropwise to the surface of the eye when used;
[0078] B, Conbercept eye drops (prepared according to Example 3) 0.1 mg / ml, colorless transparent liquid, 800 μl / bottle, batch number: 20111001, stored at 2-8°C, added dropwise to...
Embodiment 3
[0093] Embodiment 3, the preparation of Conbercept (FP3 fusion protein) eye drops
[0094] prescription:
[0095] FP3 fusion protein 10mg / ml, 9mg / ml, 5mg / ml, 1mg / ml, 0.5mg / ml, 0.1mg / ml, 0.05mg / ml or 0.01mg / ml
[0096]
[0097]
[0098] Preparation method: After thawing the concentrated FP3 fusion protein stock solution after changing the liquid, in a clean bench (sterile cabinet) of class C, add filter-sterilized solution containing 55mM citric acid, 12.5% sucrose, 250mM Arginine and 0.05% Tween 20 buffer, filter, adjust FP3 fusion protein to 10mg / ml, 9mg / ml, 5mg / ml, 1mg / ml, 0.5mg / ml, 0.1mg / ml, 0.05mg / ml or 0.01mg / ml, pH 7.5-8.3, aseptically dispensed into eye drop containers and stored at 2-8°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com