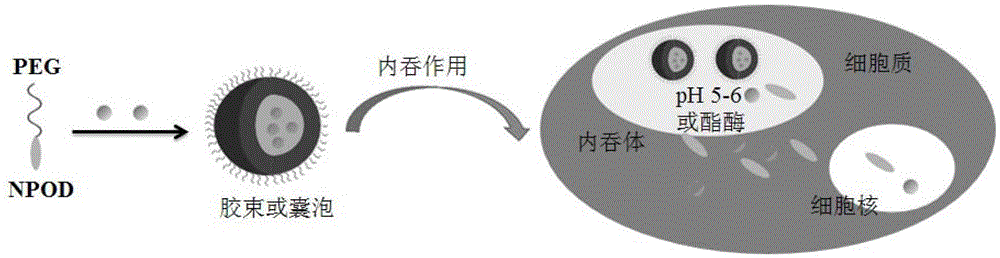
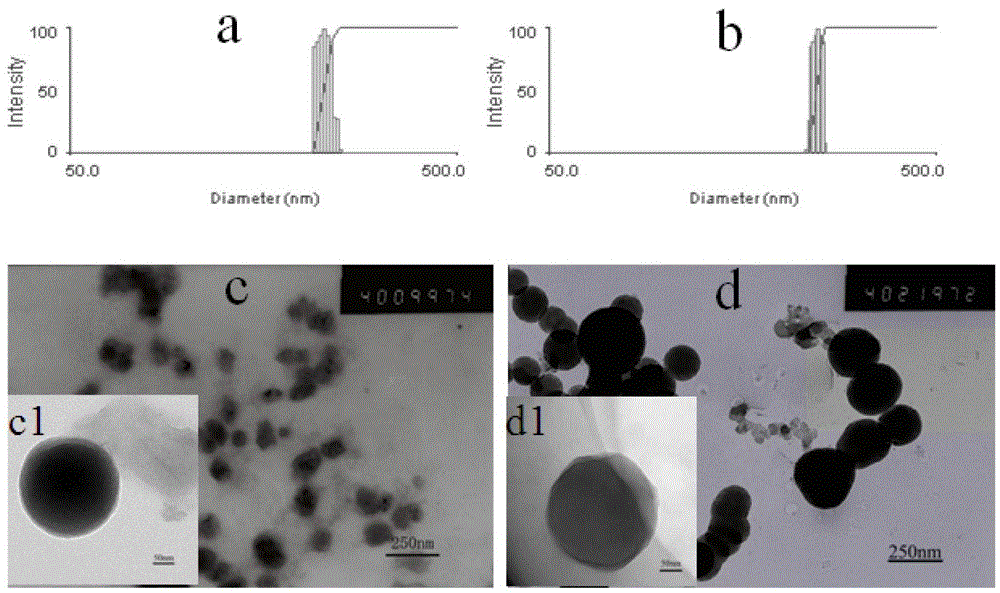
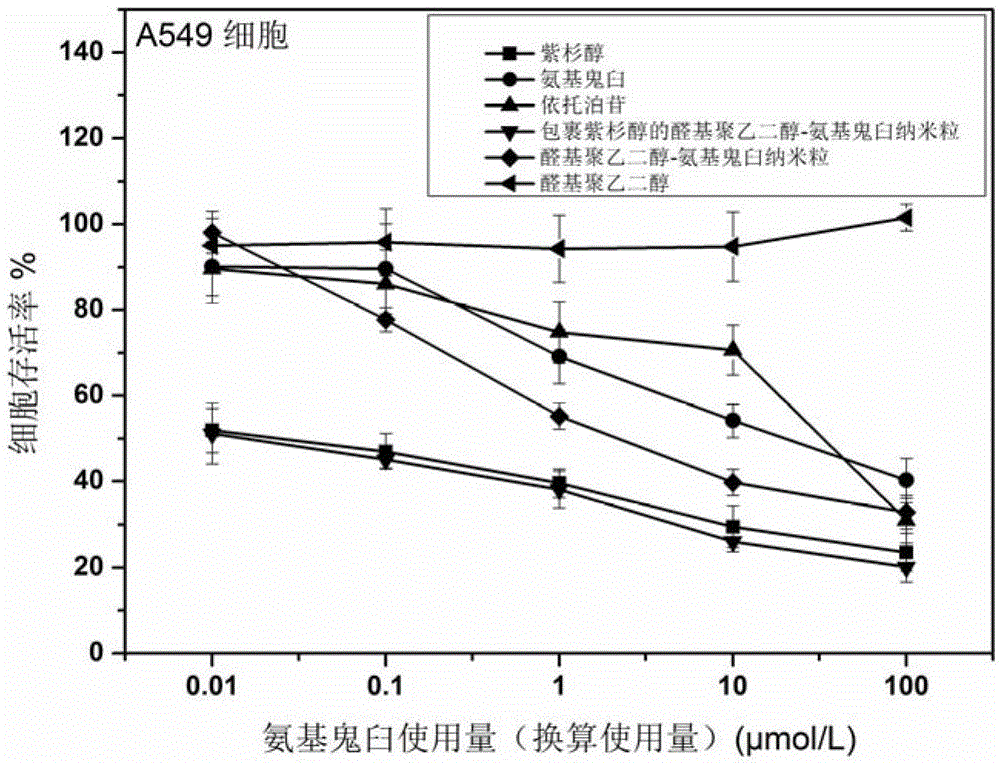
A kind of high drug-loaded podophyllin nano-prodrug and its preparation method and application
A podophyllotoxin and nano-sized technology, applied in the field of biomedicine, can solve problems such as undiscovered, achieve simple operation, simple preparation method, and improve the effect of pharmaceutical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of a PEG followed by an aminopodophyllo (NPOD) self-assembled prodrug (PEG-NPOD).
[0042] (1) Synthesis of PEG-CHO
[0043] 4.01g (5.45mmol) polyethylene glycol monomethyl ether (PEG, MW=750), 0.82g (5.46mmol) p-formylbenzoic acid and 0.33g (2.59mmol) 4- (dimethylamino) pyridine ( DMAP) was dissolved in 30ml of dichloromethane, and then 1.34g (6.54mmol) of dicyclohexylcarbodiimide (DCC) was added under ice-cooling, and the reaction solution was reacted at room temperature for 24h. After the reaction, the insoluble by-products were removed by filtration, and the filtrate was concentrated and then separated by a silica gel column. The eluent was dichloromethane / methanol 5:1, and 4.34 g of the desired product was obtained with a yield of 90.3%. The reaction formula of PEG-CHO is as follows (n=16):
[0044]
[0045] (2) Synthesis of PEG-NPOD
[0046] 1.05g (1.21mmol) of PEG-CHO was dissolved in 30ml of absolute ethanol, and then 0.59g (1.43mmol) of aminopod...
Embodiment 2
[0049] Synthesis of a self-assembled prodrug (PEG-NPOD) of one PEG followed by two aminopodophyllos (NPOD).
[0050] (1) Protection of 2,2-dimethylolpropionic acid
[0051] Dissolve 5.05g (37.28mmol) of 2,2-dimethylolpropionic acid, 7.0ml (55.91mmol) of 2,2-dimethoxypropionate and 0.355g (1.87mmol) of p-toluenesulfonic acid in 30ml of acetone , and then stirred at room temperature for 12 h. After the reaction, 0.5ml of ammonia water and ethanol solution (volume ratio 1:1) was added, and the reaction solvent was removed by rotary evaporation. The obtained solid was dissolved in 80ml of dichloromethane, and extracted twice with water (10ml each time), and separated to obtain The organic phase was dried with anhydrous sodium sulfate, and finally the solvent was removed to obtain 8.21 g of the product with a yield of 72.5%. The reaction formula is as follows:
[0052]
[0053] (2) Reaction of the protected product of 2,2-dimethylolpropionic acid with PEG:
[0054] 5.00g (6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com