Method for preparing pyrrolidine derivative
A compound, selected technology, applied in the direction of organic chemistry, etc., can solve problems such as limited applicability, and achieve the effects of good functional group compatibility, high product yield, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
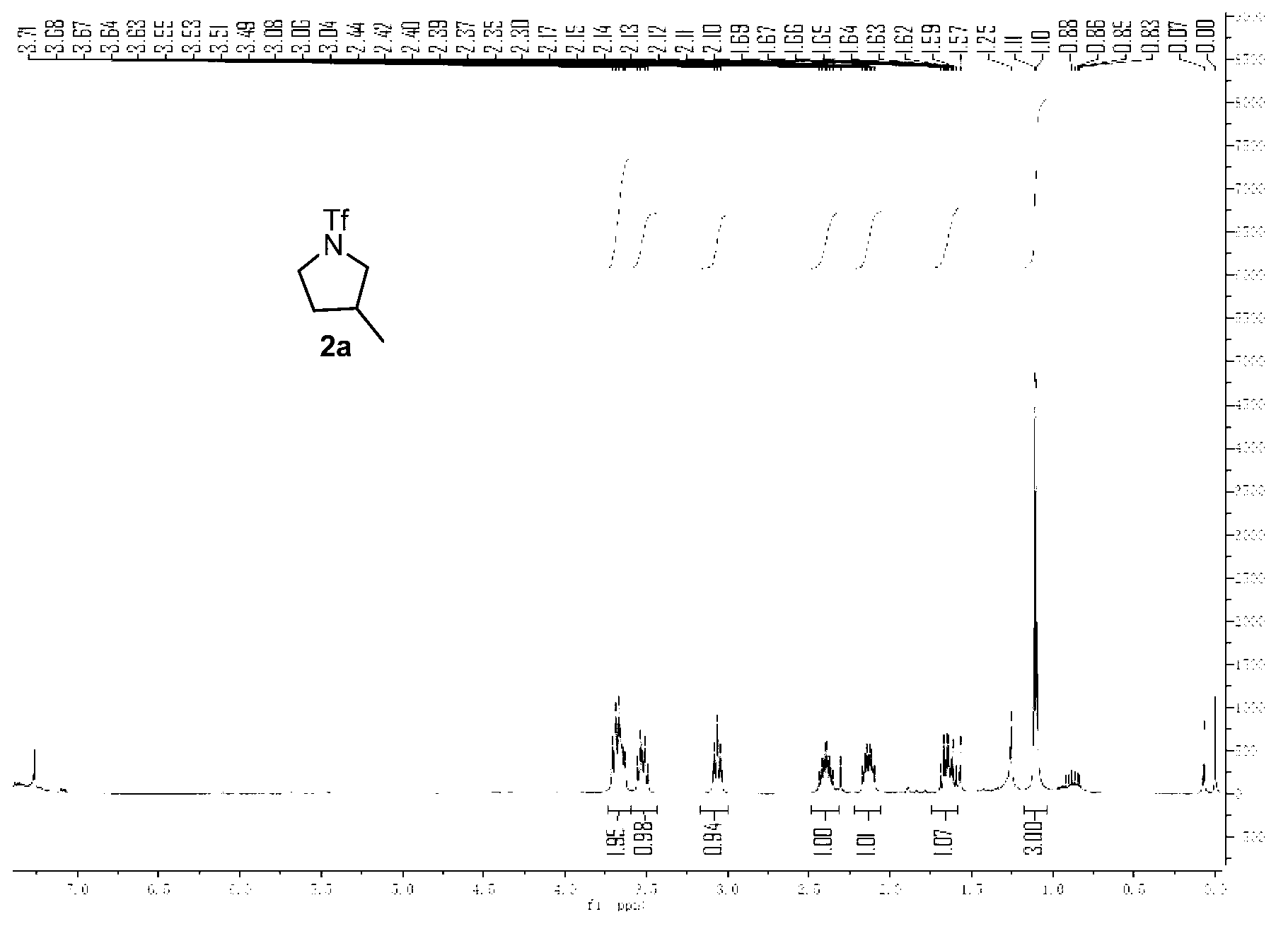
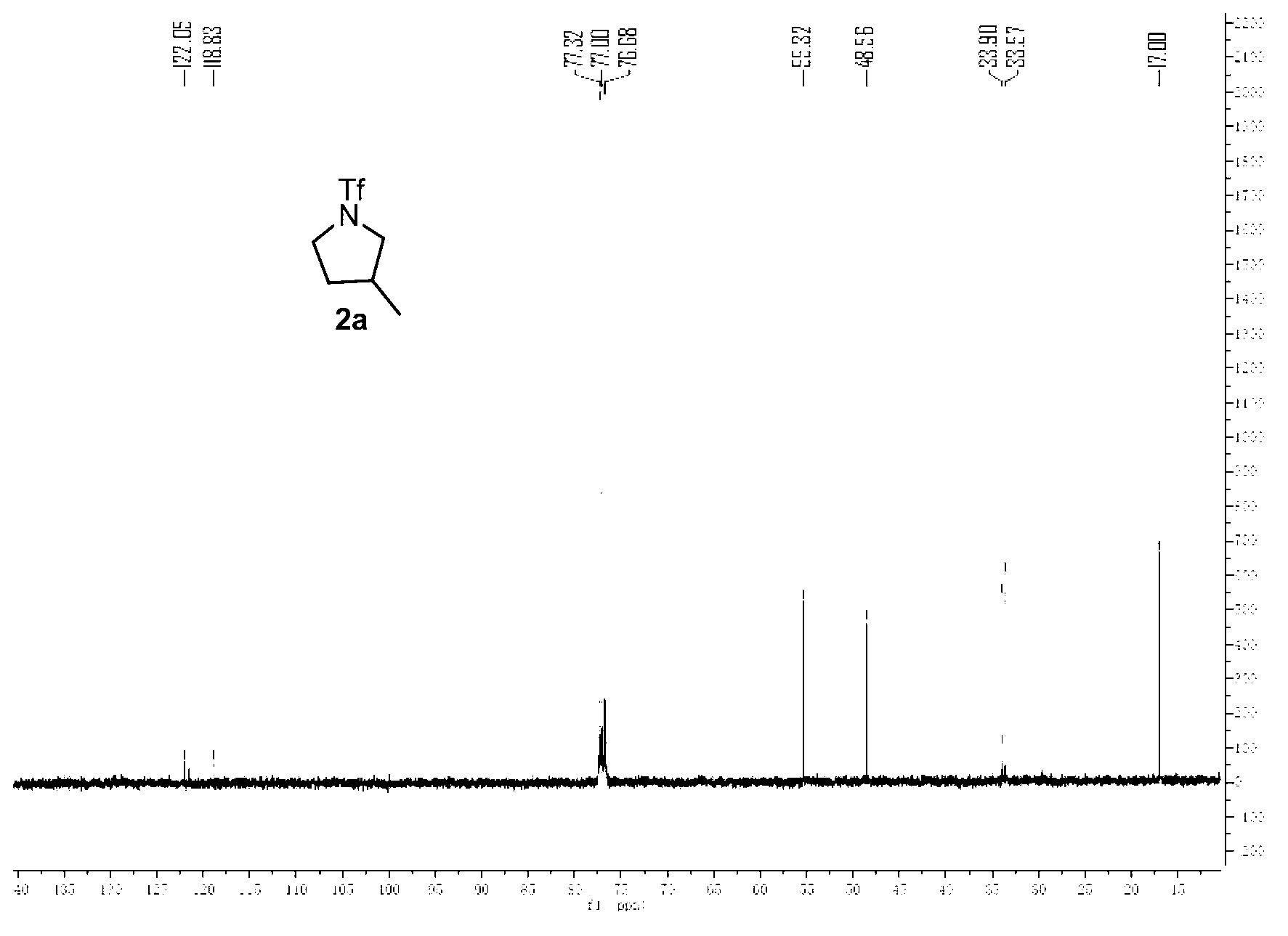
[0032] Embodiment 1, the preparation of compound 2a3-methyl-N-trifluoromethanesulfonyl tetrahydropyrrole
[0033]
[0034] Weigh 0.022g (0.10mmol) ammonia substrate trifluoromethanesulfonamide (1a) shown in formula II, 0.066g (0.20mmol) oxidant iodobenzene acetate, 0.019g (0.20mmol) alkali potassium acetate and 0.004g (0.40mmol) ligand acetylacetone and 0.017g (0.10mmol) catalyst silver acetate, mixed with 1.0mL solvent 1,2-chloroethane, injected into the system by syringe, heated to 120°C on Wattecs Reactor The intramolecular hydrogenation ring formation reaction was carried out with vigorous stirring for 12 hours. After the reaction was completed, the crude product was directly subjected to column chromatography (washing with 100mL sherwood oil, then washing with sherwood oil, dichloromethane, and a mixed solvent of ether (petroleum ether: dichloromethane: diethyl ether=60:6: 1, volume ratio) to obtain the target product 2a (yield 70%).
[0035] The structural confirmat...
Embodiment 2
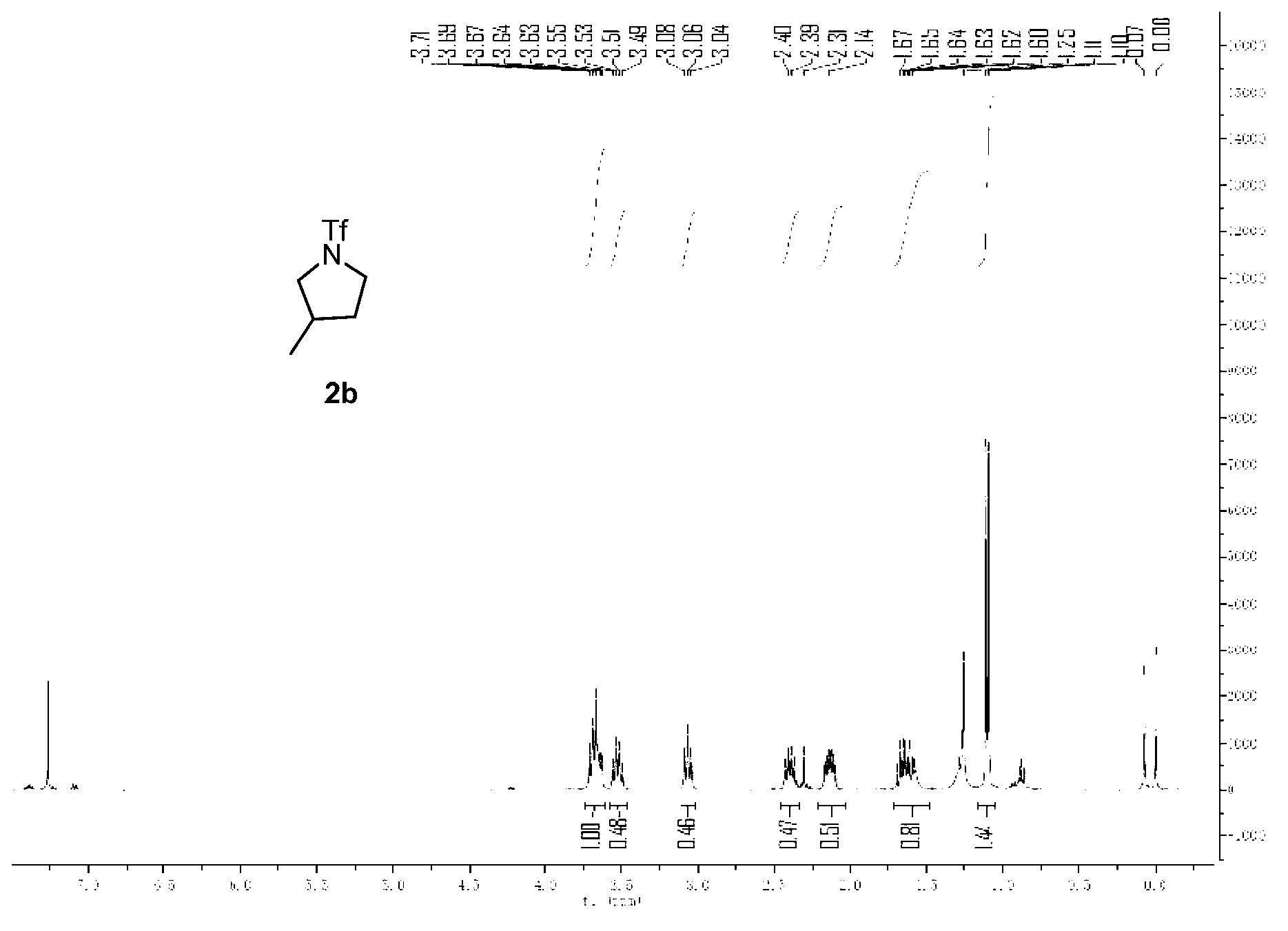
[0041] Embodiment 2, the preparation of compound 2b
[0042]
[0043] Ammonia substrates, silver acetate, iodobenzene acetate, potassium acetate, acetylacetone, 1,2-dichloroethane.
[0044] Weigh 0.022g (0.10mmol) ammonia substrate trifluoromethanesulfonamide (1b) shown in formula II, 0.066g (0.20mmol) iodobenzene acetate, 0.019g (0.2mmol) potassium acetate and 0.004g in reaction tube (0.40mmol) acetylacetone and 0.017g (0.10mmol) silver acetate, mixed with 1.0mL 1,2-chloroethane, injected into the system from a syringe, heated to 120°C on a Wattecs Reactor and stirred vigorously for 2 hours, let After the reaction tube was cooled to room temperature, 0.066 g of iodobenzene acetate was added to the reaction tube, and then heated to 120 ° C on the Wattecs Reactor, and the reaction was vigorously stirred for 3 hours. After the reaction was completed, the crude product was directly subjected to column chromatography (first with 100 mL petroleum Rinse with ether, and then rinse ...
Embodiment 3
[0051] Embodiment 3, the preparation of compound 2c
[0052]
[0053] Ammonia substrates, silver acetate, iodobenzene acetate, potassium acetate, 1,2-dichloroethane.
[0054] Weigh 0.022g (0.10mmol) ammonia substrate trifluoromethanesulfonamide (1b) shown in formula II, 0.066g (0.20mmol) iodobenzene acetate, 0.019g (0.2mmol) potassium acetate and 0.004g in reaction tube (0.40mmol) acetylacetone and 0.017g (0.10mmol) silver acetate, mixed with 1.0mL 1,2-chloroethane, injected into the system from a syringe, heated to 120°C on a Wattecs Reactor and stirred vigorously for 2 hours, let After the reaction tube was cooled to room temperature, 0.066 g of iodobenzene acetate was added to the reaction tube, and then heated to 120 ° C on the Wattecs Reactor, and the reaction was vigorously stirred for 3 hours. After the reaction was completed, the crude product was directly subjected to column chromatography (first with 100 mL petroleum Rinse with ether, and then rinse with a mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com