Biosynthetic gene cluster of grincamycin and P-1894B and application thereof
A technology of P-1894B and gricomycin, applied in the fields of plant genetic improvement, application, microorganisms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Extraction of bulk genomic DNA from Streptomyces lusitanus SCSIO LR32, which produces gricomycin and P-1894B:
[0098] The spores of fresh Streptomyces lusitanus SCSIO LR32 were inoculated into 50 mL of TSB medium (17 g of tryptone, 3 g of plant peptone, 5 g of sodium chloride, 2.5 g of dipotassium hydrogen phosphate, 2.5 g of glucose, and added water to 1L, pH7.2-7.4), 28-30°C, shake culture for about 2 days, centrifuge at 4000rpm for 10 minutes to collect mycelia. Mycelium was washed twice with STE solution (NaCl75mM, EDTA25mM, Tris-Cl20mM, the balance was water), and 30mL of STE solution and lysozyme with a final concentration of 3mg / mL were added to the washed mycelia, vortexed evenly, Incubate at 37°C for 3 hours, add proteinase K to a final concentration of 0.1-0.2mg / mL, mix well, incubate at 37°C for 10 minutes, add SDS to a final concentration of 1-2%, mix well, put in a water bath at 55°C for about 1 hours, the period is reversed several times. Add an equal v...
Embodiment 2
[0100] Establishment of Streptomyces lusitanus SCSIO LR32 Genomic Library of Grecomycin and P-1894B Producer:
[0101] First, the amount of restriction endonuclease Sau3A I was determined through a series of dilution experiments. In a 50 μL system, 5 μL of Streptomyces lusitanus SCSIO LR32 genomic DNA, 5 μL of 10× reaction buffer and a dilution of 7.5 μL were 10 -3 For Sau3A I, stop the reaction with 2.5 μL of 15 mM EDTA and the appropriate loading buffer. On this basis, a genomic DNA fragment slightly larger than 40kb was obtained by a large number of partial enzyme digestions, and dephosphorylated with a dephosphorylase.
[0102]The vector SuperCos l plasmid used to construct the library was first cut from the middle of the two cos sequences with restriction endonuclease Xba I, then dephosphorylated, and then used restriction endonuclease Bam from the multiple cloning site HI is cut to obtain two arms. The treated vector was ligated with the previously prepared partially ...
Embodiment 3
[0105] Screen positive clones containing biosynthetic genes of gramamicin and P-1894B from the genome library of Streptomyces lusitanus SCSIO LR32, which produces grammycin and P-1894B:
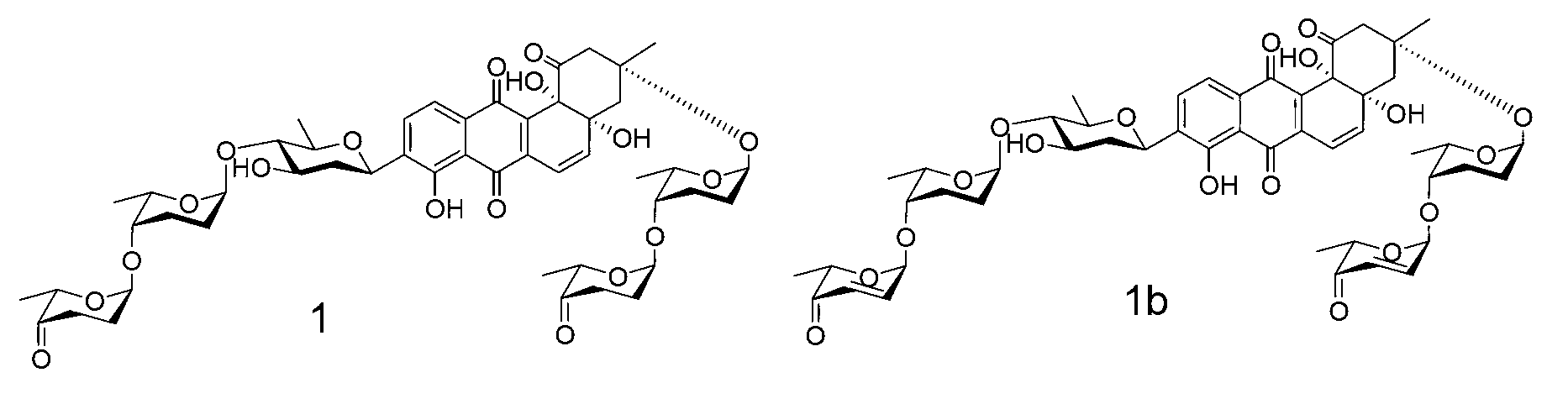
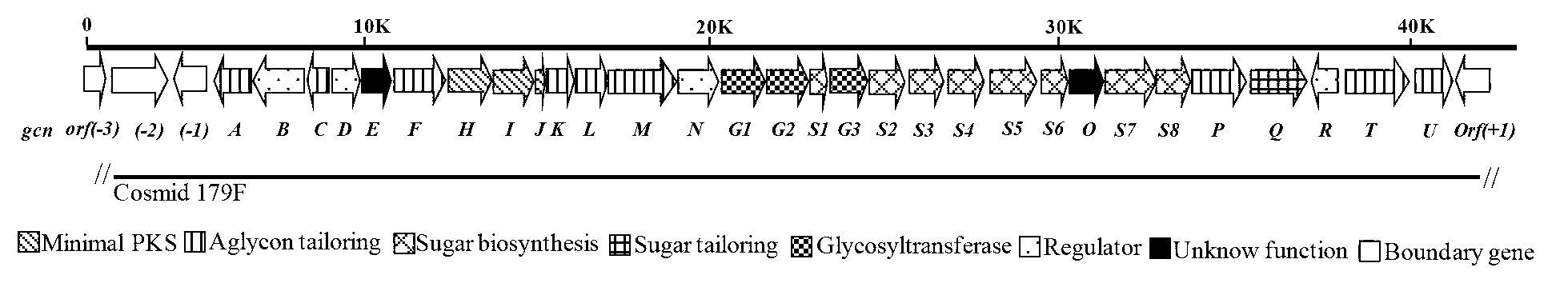
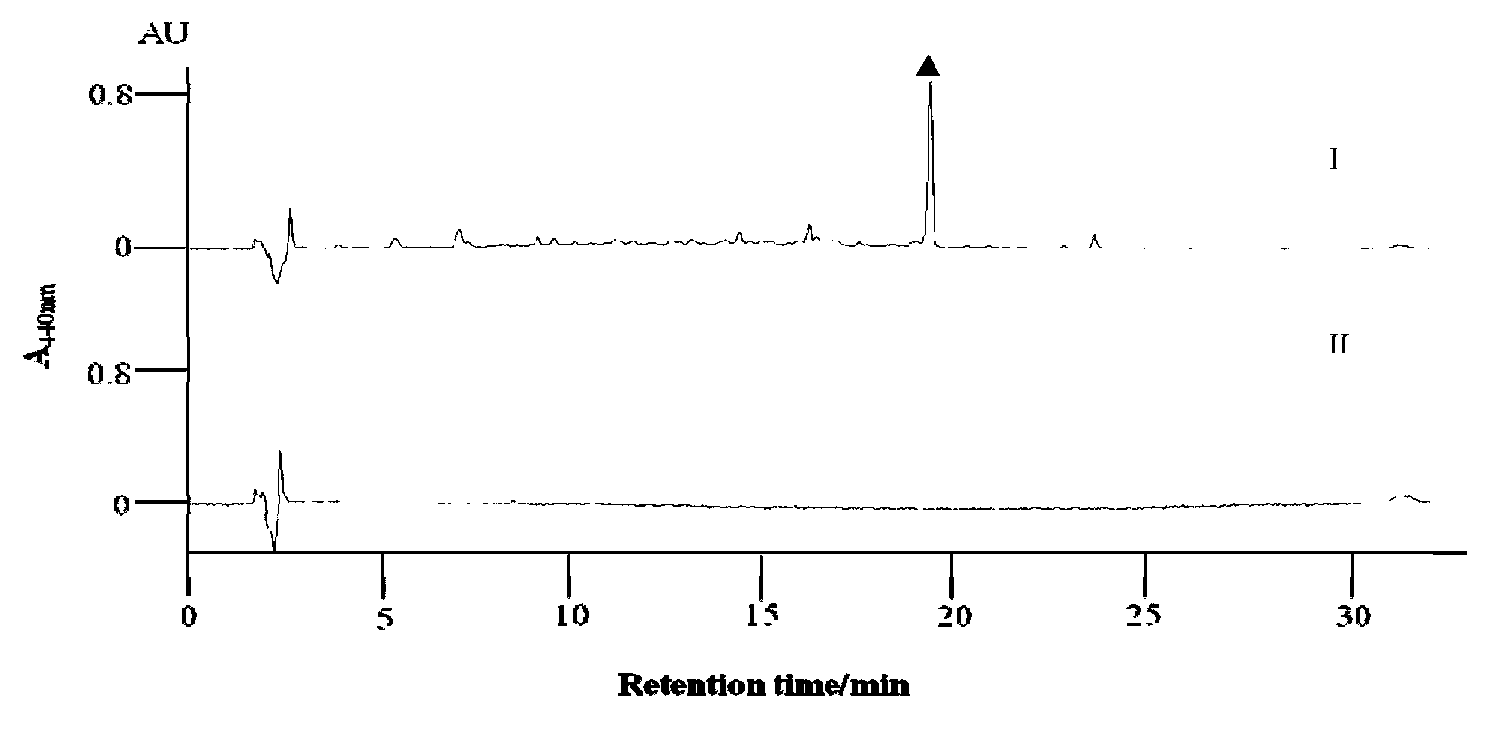
[0106] By analyzing the structure of gricomycin and P-1894B and the reports of related literature, 2,3-dehydratase gene was used as screening primer (Table 2) for PCR screening, and 10 positive clones were obtained from 2300 clones , determine the positive clones containing the biosynthetic gene cluster of gramamicin and P-1894B, and perform sequencing, and one of them contains the positive clone of the entire biosynthetic gene cluster of gramamicin and P-1894B— The nucleotide sequence of cosmid179F is shown in SEQ ID NO.1, and the nucleotide sequence of the biosynthetic gene cluster of gricomycin and P-1894B is shown in the 3722-40612 position of the sequence shown in SEQ ID NO.1 The base sequence is shown.
[0107] Table 2: Library Screening Primers
[0108]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com