Tri-indene-pyrene derivative blue-light emitting material, and preparation method and application thereof
A technology of pyrene derivatives and blue light materials, which is applied in the direction of luminescent materials, chemical instruments and methods, hydrocarbons, etc., can solve the problems of difficult preparation and mass production, and many steps in synthesis, so as to reduce quenching effect and synthesize The effect of simple process and high-efficiency electro-induced blue light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
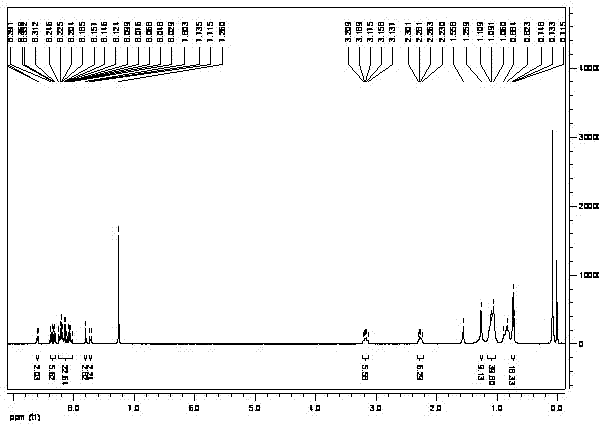
[0025] Synthesis of Triindene-Pyrene Derivative TrPy
[0026]
[0027] Target compound TrPy
[0028] According to the reaction route, compound 1-bromopyrene (1) is dissolved in freshly distilled tetrahydrofuran solvent, reacts with butyllithium at -78°C, and then adds compound pinacol borate 2 to obtain compound 1- Pyrene borate 3. Finally, 1-pyrene boronic acid ester 3 and tribromotriindene core 4 undergo Suzuki coupling reaction under the condition of tetrakistriphenylphosphopalladium as catalyst to obtain the target compound TrPy.
[0029] 【Reaction route】
[0030]
[0031]
Embodiment 2
[0033] Fabrication of Organic Electroluminescent Devices as Emissive Layers
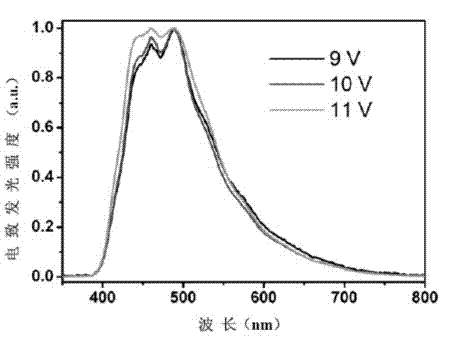
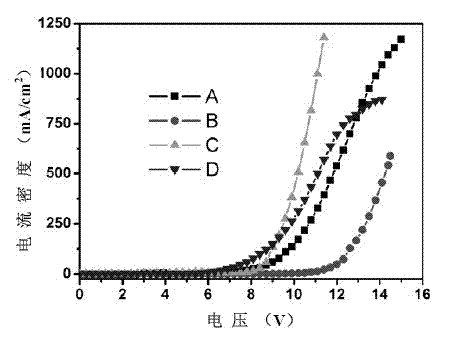
[0034] A blue light device A, the device structure is: ITO / PEDOT:PSS (20nm) / NPB (30nm) / TrPy (30nm) / TPBI (40nm) / Ca:Ag; wherein TrPy is a blue light material of three indene-pyrene derivatives , as a light-emitting layer, and prepared by evaporation.
Embodiment 3
[0036] Fabrication of Organic Electroluminescent Devices as Emissive Layers
[0037] A blue light device B, the device structure is: ITO / PEDOT:PSS(20nm) / NPB(30nm) / TrPy(30nm) / BAlq(40nm) / Ca:Ag; wherein TrPy is a triacene-pyrene derivative blue light material , as a light-emitting layer, and prepared by evaporation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com