Azilsartan preparation method
A compound and reaction technology, applied in the field of medicine, can solve the problems such as low yield of Azilsartan, unfavorable industrial production, and many deethylated impurities, and achieve the effects of shortening reaction time, reducing content and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
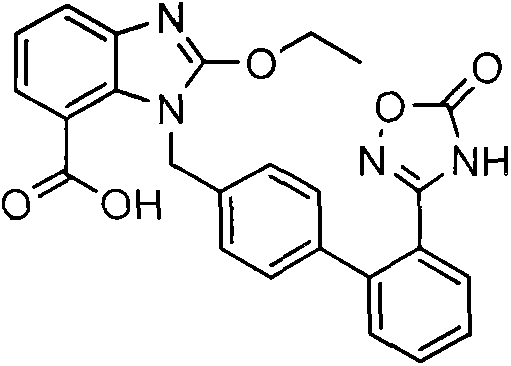
[0037] Embodiment 1: Preparation of Azilsartan
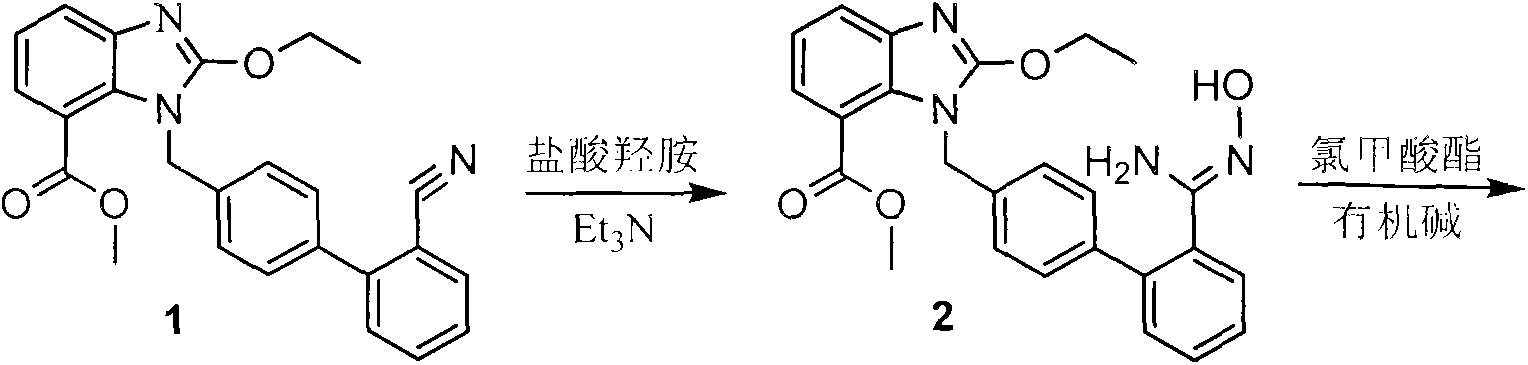
[0038] The first step: preparation of compound 2
[0039] Add 20g of compound 1 to a 500mL four-neck flask, add 10.14g (3eq) of hydroxylamine hydrochloride in 100mL of dimethyl sulfoxide solution under stirring, heat to 90°C, add 30.95g (6eq) in batches under stirring ) sodium carbonate, add and keep warm for 9-10 hours, after the reaction is completed, cool down to room temperature, add 100mL of water and stir, the solid precipitates, cool down to 0-5°C, continue to stir for about 1h, filter and dry to obtain 15.2g of compound 2, the yield was 70.4%.
[0040] The second step: the preparation of compound 3
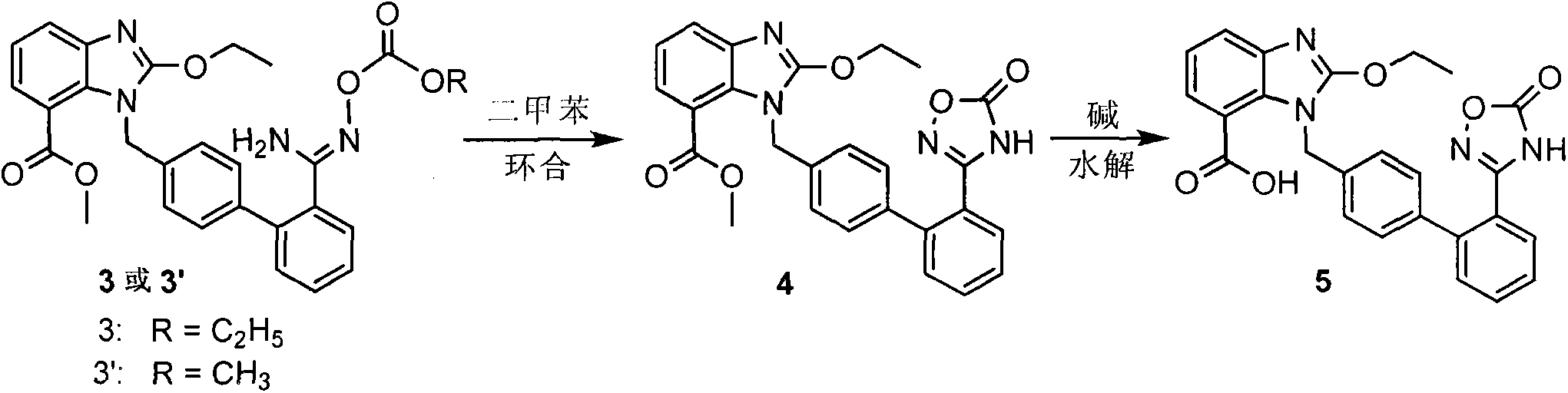
[0041] Add 10g of compound 2 to a 500mL four-neck flask, add it into 100mL tetrahydrofuran solution under stirring, cool down to 0-5°C, add 6.3mL triethylamine, add dropwise tetrahydrofuran dissolved in 3mL ethyl chloroformate (1.2eq) Solution, after dripping, keep warm for about 1h. After the reaction was completed, 100...
Embodiment 2
[0044] Embodiment 2: the preparation of Azilsartan
[0045] The preparation of compound 3 was as described in Example 1; 10 g of compound 3 was added to a 250 mL three-neck flask, and added to a mixed solution of 50 mL of tetrahydrofuran and 50 mL of water under stirring conditions, and 2.4 g of lithium hydroxide monohydrate was added, and kept for 50- 60°C, react for about 15h. After the reaction was completed, the temperature was lowered to 20-25°C, the pH was adjusted to 1-2 with 2N hydrochloric acid, and a solid was precipitated, kept stirring for 1-2 hours, filtered with suction, and dried to obtain 8.4 g of off-white solid, namely azilsartan, with an HPLC purity of 99.70 %, the yield is 95.0%.
Embodiment 3
[0046] Embodiment 3: the preparation of azilsartan
[0047] The preparation of Compound 3 was the same as described in Example 1; a mixed solution of 50 mL of tetrahydrofuran and 50 mL of water was added to a 250 mL three-neck flask, 10 g of Compound 3 was weighed and added to the reaction flask under stirring, 3.3 g of potassium hydroxide was added, and the temperature was kept for 40 React at -50°C for about 9-10h. After the reaction, cool down to 20-25°C, adjust the pH to 1-2 with 2N hydrochloric acid, and precipitate a solid, keep stirring for 1-2h, filter with suction, and dry to obtain 8.1g of off-white solid, namely Azilsartan, the HPLC purity is 99.55%, and the yield is 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com