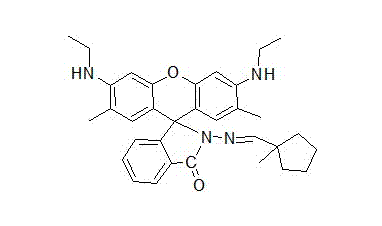
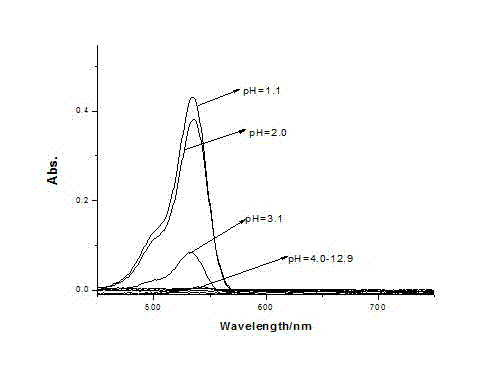
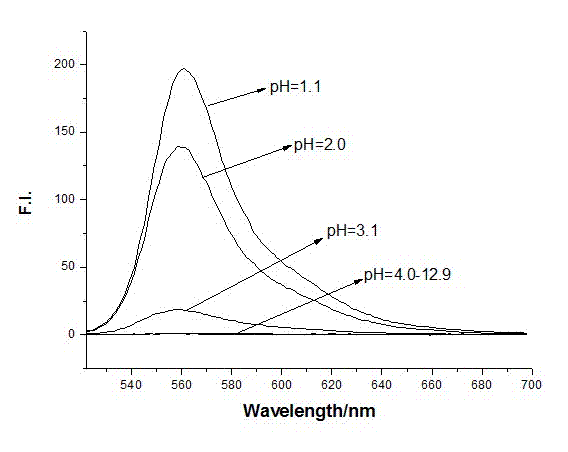
P-N-methyl cyclopentaldehyde rhodamine 6G pH fluorescence molecular probe as well as preparation method and use thereof
A technology of methylcyclopentanal and iminomethyl, which is applied in the application field of chemical sensing technology, can solve the problems of high impedance of glass electrodes, low sensitivity of colorimetry, and can not meet the detection requirements, and achieves high sensitivity and superior spectral performance. , the effect of short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] compound Synthesis:
[0034] (1) Synthesis of raw material rhodamine 6G hydrazide
[0035] Dissolve 2.0 g of Rhodamine 6G in 30 mL of absolute ethanol, add it to a 100 mL flask, add 2.8 mL of 80% hydrazine hydrate to the flask at room temperature, stir for 30 minutes, and then heat the mixed system to reflux, when the color of the solution The reaction was completed when the color changed from scarlet to clear orange; cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure to obtain 1.7 g of the product with a yield of 88.3%.
[0036] (2) Synthesis of the target compound
[0037] Add 1.0 g of rhodamine 6G hydrazide prepared above and 0.25 g of 1-methylcyclopentaldehyde to a 100 mL flask, add 6 mL of anhydrous methanol to dissolve the solid, then add 5 drops of glacial acetic acid dropwise, and heat the mixed system Reflux to complete reaction, after the reaction is completed, drop to room temperature, pour the mixed syst...
Embodiment 2
[0043] compound Synthesis:
[0044] (1) Synthesis of raw material rhodamine 6G hydrazide
[0045] Dissolve 5.0 g of Rhodamine 6G in 100 mL of absolute ethanol, add it to a 250 mL flask, add 9.6 mL of 80% hydrazine hydrate to the mixed system at room temperature, stir for 30 minutes, then heat the mixed system to reflux, when the color of the solution When it changed from scarlet to clear orange, the reaction was completed; the system was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure to obtain 3.8 g of the product with a yield of 78.4%.
[0046] (2) Synthesis of the target compound
[0047] Add 2.0 g of rhodamine 6G hydrazide and 0.5 g of 1-methylcyclopentaldehyde to a 100 mL flask, add 20 mL of anhydrous methanol to dissolve the solid, then add 0.2 mL of glacial acetic acid, heat the mixed system to reflux, and wait for the reaction After completion, the mixed system was lowered to room temperature, the mixed system in...
Embodiment 3
[0049] compound Synthesis:
[0050] (1) Synthesis of raw material rhodamine 6G hydrazide
[0051] Dissolve 10.0 g of Rhodamine 6G in 198 mL of absolute ethanol, add it to a 250 mL flask, add 16.0 mL of 80% hydrazine hydrate to the mixed system at room temperature, stir for 30 minutes, then heat the mixed system to reflux, when the color of the solution When the color changed from scarlet to clear orange, the reaction was complete; the mixture was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure to obtain 6.8 g of the product, with a yield of 71.0%.
[0052] (2) Synthesis of the target compound
[0053] Add 5.0 g of rhodamine 6G hydrazide and 1.3 g of 1-methylcyclopentaldehyde to a 100 mL flask, add 40 mL of anhydrous methanol to dissolve the solid, then add 0.5 mL of glacial acetic acid, and heat the mixed system to reflux until complete reaction , down to room temperature after the completion of the reaction, pour the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com