High-activity bifunctional catalyst for preparing chiral epoxy alkane and diol and application thereof
A bifunctional catalyst and alkylene oxide technology, which is applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, hydrolysis preparation, etc., can solve the problem of prolonging reaction time, reducing reaction efficiency, poor solubility, etc. problem, to achieve the effect of high product enantioselectivity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
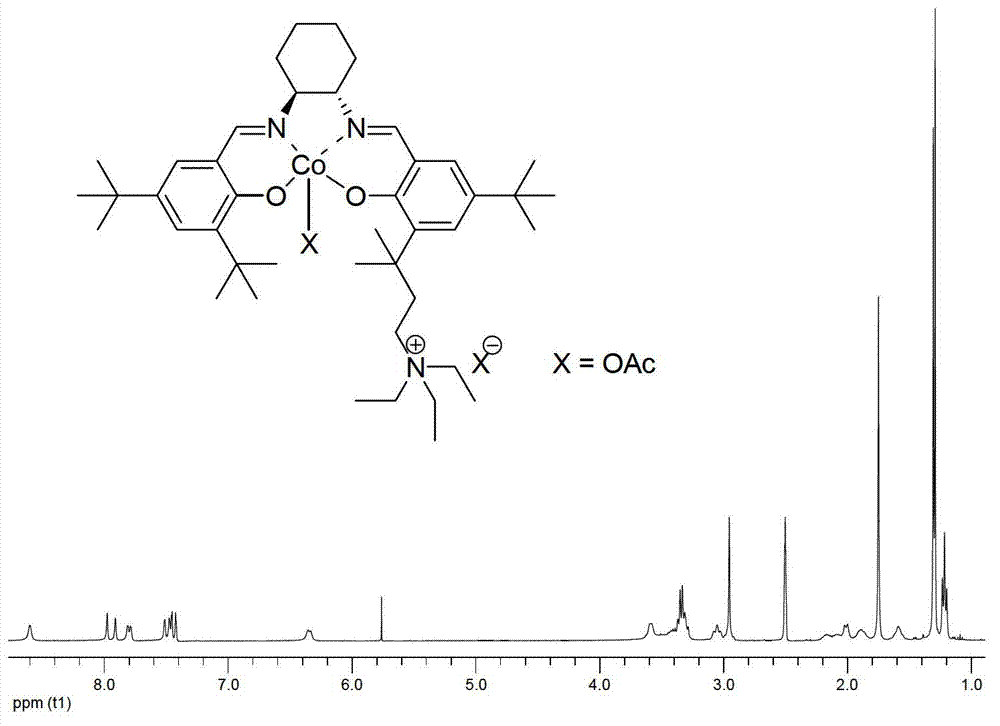
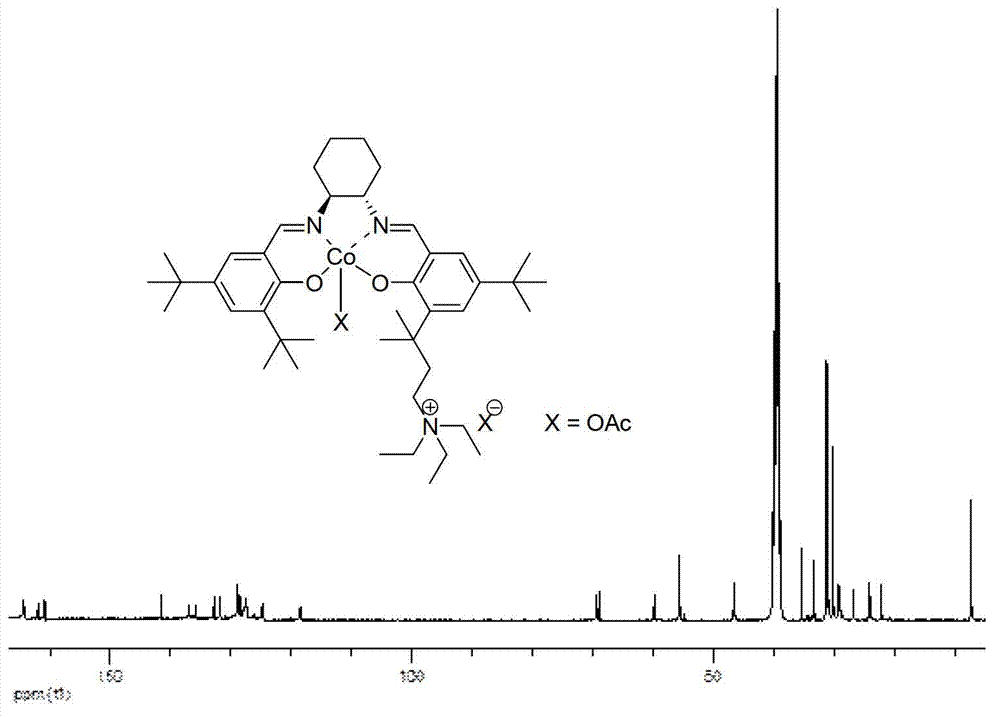
[0036]
[0037] Compound b: In a 100mL round bottom flask, dissolve compound a (3.60g, 10.0mmol) and anhydrous potassium carbonate (1.5g, 12.0mmol) in 50mL refined acetonitrile, add refined diethylamine (1.7mL, 12.0mmol), Reacted at 80°C for 24 h, followed the reaction by TLC until no raw materials remained, stopped the reaction, cooled the reaction liquid to room temperature, filtered, and removed the solvent under reduced pressure to obtain the crude product as a white turbid liquid. Dissolve it in 10ml of ethyl acetate, add 40mL of 2mol / L dilute hydrochloric acid solution, and stir vigorously for 0.5h. Separate the aqueous phase, extract the organic phase with water (20mL×3), combine the aqueous phases, slowly add saturated sodium bicarbonate solution to make it alkaline to pH=8, and extract with dichloromethane (50mL×3). The combined organic phases were washed with saturated brine (200 mL×1), dried over anhydrous sodium sulfate, and the solvent was removed under reduced p...
Embodiment 2
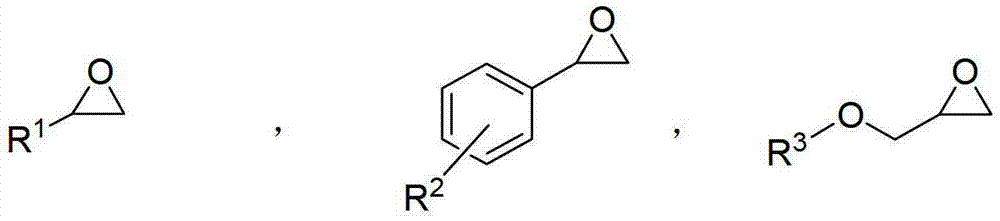
[0055] Add alkylene oxide and the prepared catalyst in a flask with a volume of 200mL, and after cooling to 0°C, slowly add water dropwise. , stop responding. A small amount of the reaction solution was taken for NMR analysis. Distill unreacted alkylene oxide under normal pressure or reduced pressure, weigh and calculate the yield, and analyze its optical purity with gas phase or liquid chromatography; carry out vacuum distillation on the residual liquid to obtain diol products, weigh and calculate the yield, and use gas phase or liquid chromatography to analyze its optical purity; Or liquid chromatography to analyze its optical purity.
[0056] The results are shown in Table 1-6.
[0057] Table 1 Table 2 table 3 Table 4
[0058] table 5 Table 6
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com