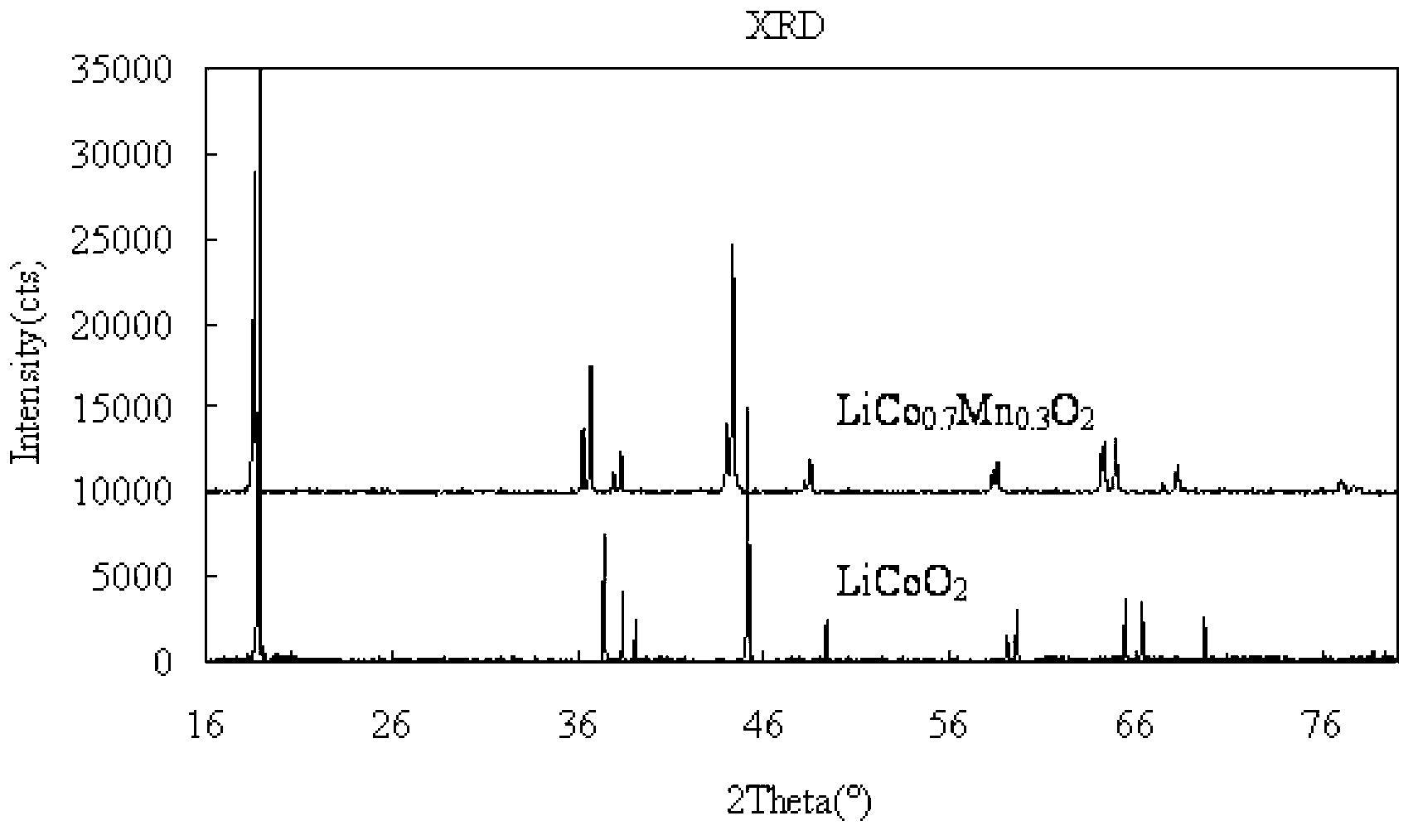
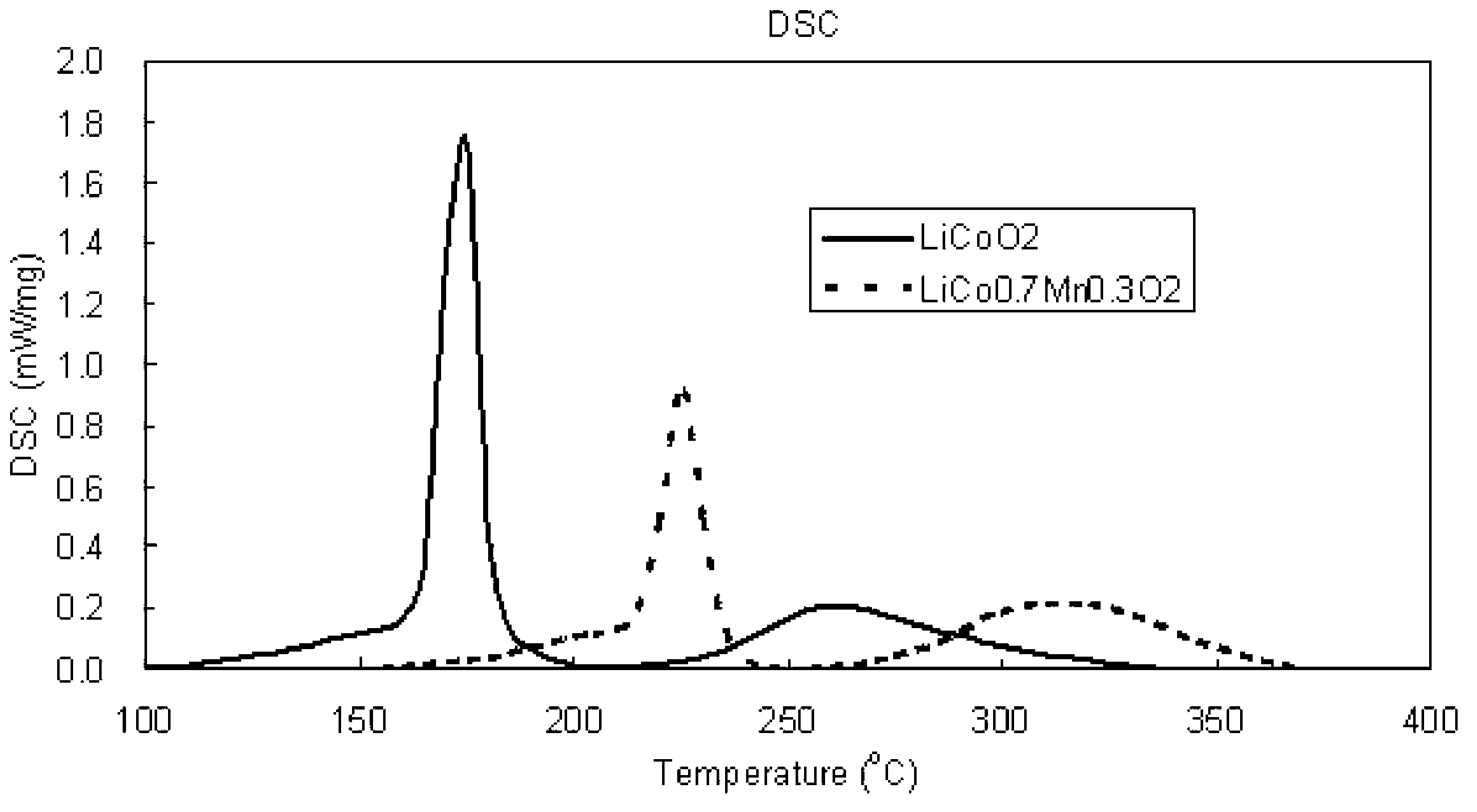
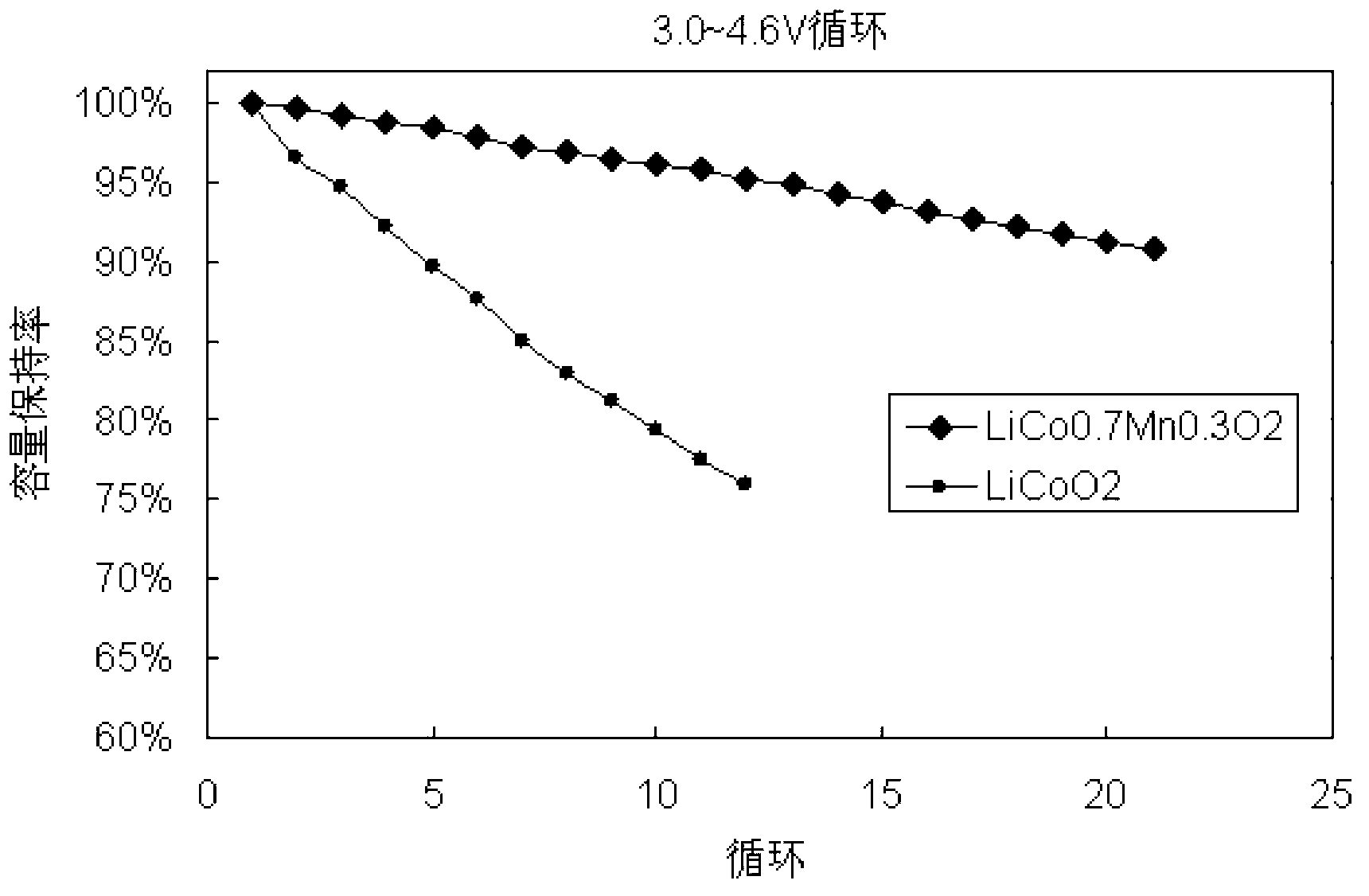
Polymorph positive electrode material for lithium ion battery and preparation method of material
A technology for lithium ion batteries and cathode materials, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of cycle performance decline, cannot effectively solve the irreversible changes of lithium cobalt oxide, etc., and achieve the effect of improving the surface structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve manganese nitrate in water, add ammonium citrate, and stir evenly to form a solution with a Mn concentration of 1mol / L, wherein the molar ratio Mn:NH 4 + =1:1; Dissolve cobalt acetate in water to form a 1mol / L solution; configure 1mol / L LiOH and ammonia solution; make the above three solutions according to the molar ratio of Mn:Co:Li of 0.3:0.7:1 at the same time Spray into the atomization chamber, the dewdrops formed by atomization are in the range of 10-500nm, and heated and dried at 200°C to obtain LiCo 0.7 mn 0.3 (OH) 3 Precursor, sintered at 500°C to obtain LiCo 0.7 mn 0.3 o 2 .
[0036] Table 1 is LiCo 0.7 mn 0.3 o 2 with LiCoO 2 The comparison of the gram capacity under different magnifications can be seen from Table 1, because Mn replaces part of Co, LiCo 0.7 mn 0.3 o 2 Compared with LiCoO at 0.2C gram capacity 2 low, but when the discharge rate increases to 2C, LiCo 0.7 mn 0.3 o 2 The gram-to-capacity ratio of LiCoO 2 The gram capacit...
Embodiment 2
[0043] Dissolve manganese acetate and nickel nitrate in water at a molar ratio of Mn:Ni of 1:2, add diammonium edetate, and stir evenly to form a solution with a Mn concentration of 1mol / L, wherein the molar ratio Mn:Ni:NH 4 + =1:2:3; Dissolve cobalt nitrate in water to form a 1mol / L solution; configure 1mol / L LiOH and ammonia solution; make the above three solutions according to the molar ratio of Mn:Ni:Co:Li is 0.1:0.2 : 0.7: 1 and sprayed into the atomization chamber at a uniform speed at the same time. 0.7 mn 0.1 Ni 0.2 (OH) 3 precursor, and then sintered at 1100 °C to obtain LiCo 0.7 mn 0.1 Ni 0.2 o 2 .
[0044] Figure 4 Is LiCo 0.7 mn 0.1 Ni 0.2 o 2 with LiCoO 2 At 25 ℃, 3.0 ~ 4.6V, 0.5C / 0.5C charge and discharge cycle curve, it can be seen from the figure that LiCo 0.7 mn 0.1 Ni 0.2 o 2 It has ideal cycle performance.
Embodiment 3
[0046] Dissolve manganese nitrate and nickel acetate in water at a molar ratio of Mn:Ni=1:1, add ammonium citrate, and stir evenly to form a solution with a Mn concentration of 1mol / L, wherein the molar ratio Mn:Ni:NH 4 + =1:1:2; Dissolve cobalt nitrate in water to form a 1mol / L solution; configure 1mol / L LiOH and ammonia solution; make the above three solutions according to the molar ratio of Mn:Ni:Co:Li is 0.05:0.05 : 0.9: 1 and sprayed into the atomization chamber at a uniform speed at the same time, the dewdrops formed by atomization are in the range of 10-500nm, and heated and dried at 200°C to obtain LiCo 0.9 mn 0.05 Ni 0.05 (OH) 3 Precursor; sintered at 900°C to obtain LiCo with layered structure and spinel structure 0.9 mn 0.05 Ni 0.05 o 2 ; the LiCo 0.9 mn 0.05 Ni 0.05 o 2 with Al(OH) 3 According to the molar ratio Li:Al=1:0.02, mix evenly, and sinter at 600°C for 2h to obtain LiCo coated with Al. 0.88 mn 0.05 Ni 0.05 al 0.02 o 2 .
[0047] LiCo 0.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com