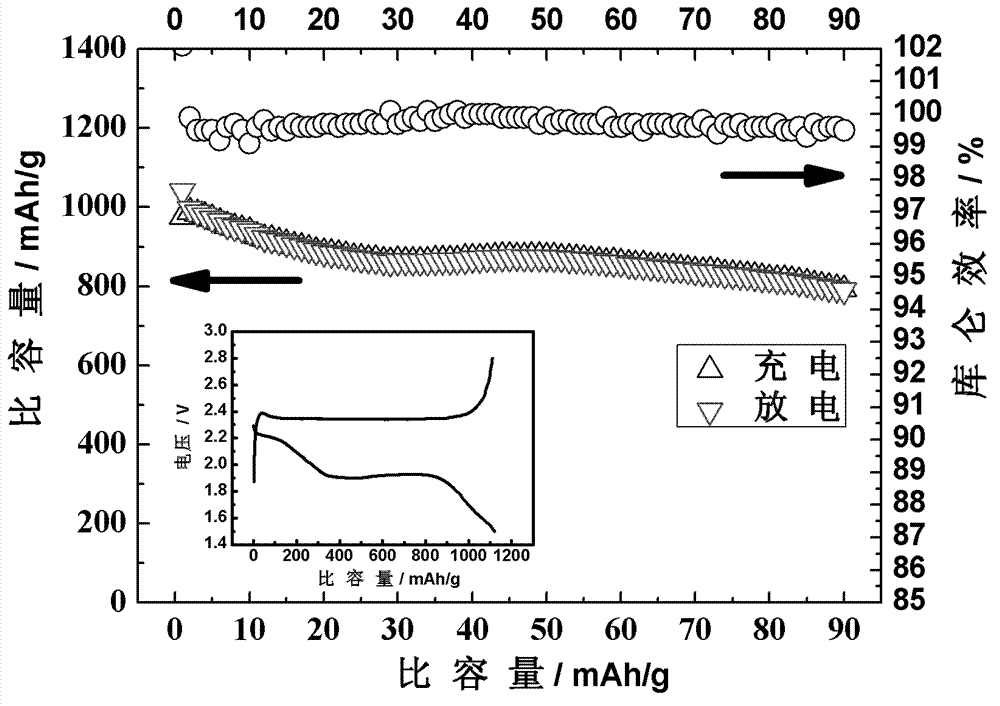
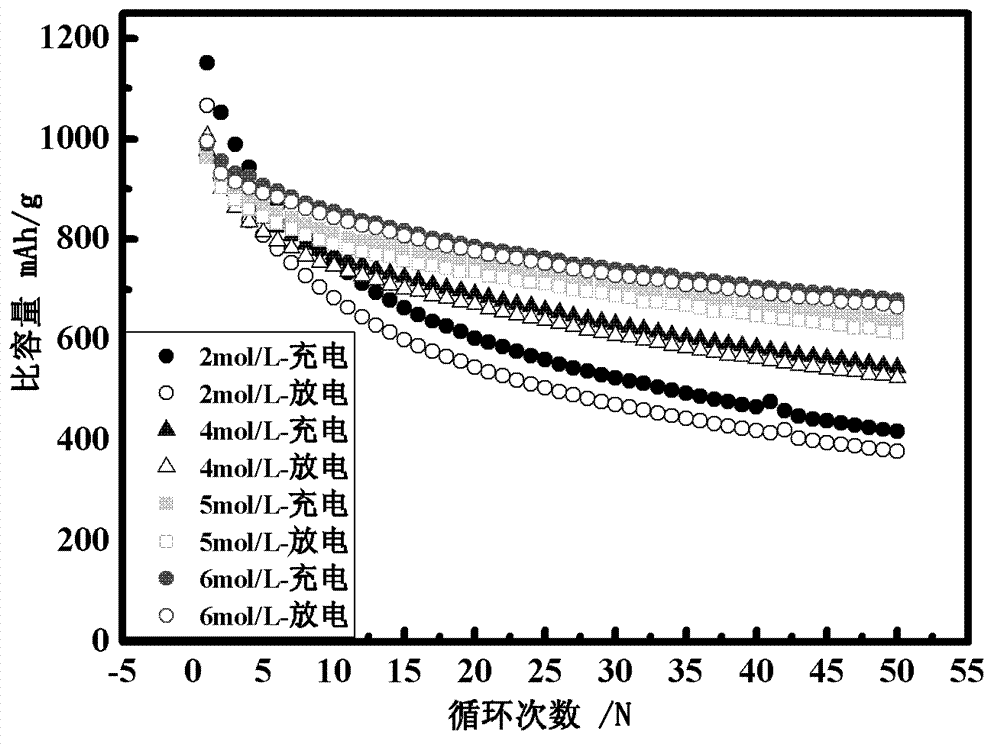
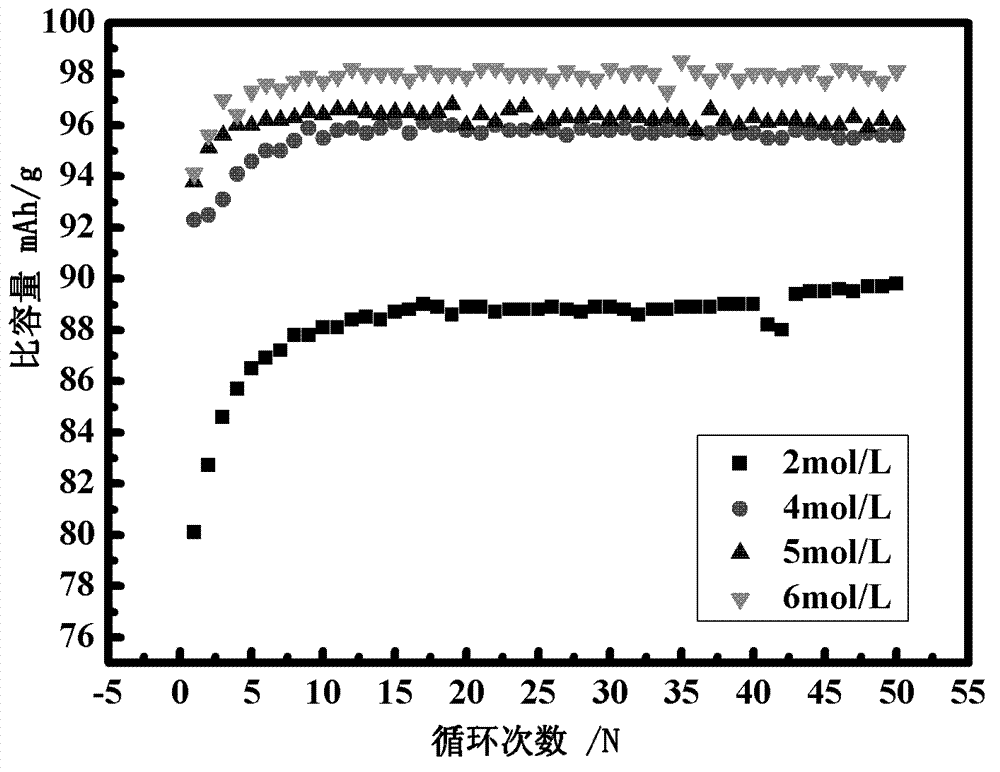
Lithium-sulfur secondary battery system
A lithium-sulfur secondary battery and system technology, applied in secondary batteries, battery electrodes, electrode carriers/current collectors, etc., can solve the problems of poor cycle performance of lithium-sulfur batteries, reduce the solubility of polysulfide ions, etc., and improve Coulombic efficiency , Improve the interface performance, the effect of good adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The lithium-sulfur battery system simulates the battery, and the specific manufacturing process is as follows:
[0021] Positive electrode material and its pole piece production process:
[0022] The carbon-sulfur composite material uses 2# porous carbon. The specific parameters are as follows: specific surface area (m2 / g): 1431, average pore diameter (nm): 3.8, pore volume (cc / g): 1.58
[0023] The preparation process of the cathode material is as follows:
[0024] Mix porous carbon 2# with elemental sulfur powder at a ratio of 4:6 by weight, seal the above carbon-sulfur composite material into an airtight argon-filled glass tube, and heat-treat the raw material at 155 degrees for 24 hours.
[0025] Weigh a certain amount of carbon-sulfur composite material, carbon nanotubes and polyvinylidene fluoride (PVDF) according to the weight percentage of 8:1:1, and stir and mix them evenly with pyrrolidone as a dispersant. The aluminum foil coated with a layer of carbon is u...
Embodiment 2
[0031] The lithium-sulfur battery system simulates the battery, and the specific manufacturing process is as follows:
[0032] Positive electrode material and its pole piece production process:
[0033] The carbon-sulfur composite material uses 1# porous carbon. The specific parameters are as follows: specific surface area (m2 / g): 671, average pore diameter (nm): 2.5, pore volume (cc / g): 0.77
[0034] The carbon-sulfur composite material uses 2# porous carbon. The specific parameters are as follows: specific surface area (m2 / g): 1431, average pore diameter (nm): 3.8, pore volume (cc / g): 1.58
[0035] The preparation process of the cathode material is as follows:
[0036] Mix porous carbon 1# with elemental sulfur powder at a weight percentage of 5:5, and porous carbon 2# and elemental sulfur powder at a weight percentage of 4:6, 3:7, and 2:8, respectively, and compound the above four kinds of carbon and sulfur The material was enclosed and sealed in an argon-filled glass tub...
Embodiment 3
[0044] The lithium-sulfur battery system simulates the battery, and the specific process is as follows:
[0045] Positive electrode material and its pole piece production process:
[0046] The preparation process of the carbon-sulfur composite material is as follows: the porous carbon 1# is mixed with sulfur powder at a weight percentage of 5:5, sealed and airtight in an argon-filled glass tube, and the raw material is treated at 155 degrees for 24 hours.
[0047] Weigh a certain amount of carbon-sulfur composite material, sodium alginate and acetylene black according to the weight percentage of 7:2:1, and use deionized water as a dispersant to stir and mix them evenly. Using aluminum foil as a current collector, the mixed slurry is evenly coated on the current collector, then dried and cut into pole pieces with the same shape and area. The negative pole piece is made of lithium metal.
[0048] Electrolyte system:
[0049] The electrolytic solution adopts an organic electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com