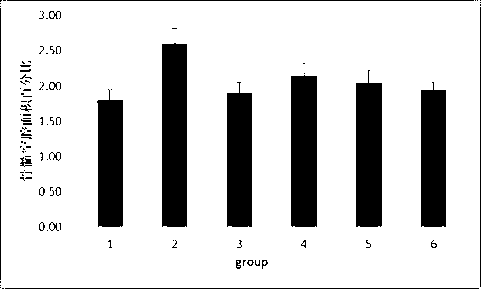
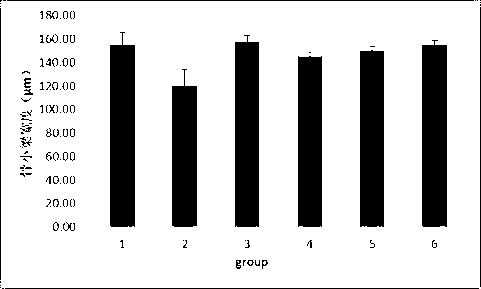
Medicinal composite containing cornu cervi pantotrichum and application thereof
A composition, the technology of velvet antler, applied in the field of medicine, can solve problems such as indigestion and complex ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Contains the medicinal composition powder of deer antler
[0024]
[0025] The velvet is selected from sika deer or red deer. When it grows for 30 days, use a pre-sterilized medical hand saw to saw off the velvet, and use a vacuum pump to draw blood and then freeze-dry; after freezing with the aid of liquid nitrogen, grind it into powder, and use normal saline or phosphate Buffer solution, extract at 4°C for 24 hours; centrifuge the extract at 4,500 rpm for 30 minutes at 4°C, take the supernatant and remove most of the insoluble matter; freeze-dry the supernatant to obtain velvet antler freeze-dried powder, and refrigerate at 4°C.
[0026] The germinated soybean powder is prepared by a method known in the art: after germinating for 2 to 3 days, the soybeans with 4-5mm sprouts are baked and crushed, and passed through a 100-mesh sieve.
[0027] The powder can be prepared through the following steps:
[0028] The above medicinal flavors are pulverized a...
Embodiment 2
[0032] Example 2 Containing medicinal composition powder of velvet antler
[0033] A medicinal composition containing deer antler, by weight: 1% velvet antler freeze-dried powder; 1% glucosamine; 1% collagen; 1% vitamin B6; 1% dolomite; 95% germinated soybean powder.
[0034] All the other are with embodiment 1.
Embodiment 3
[0036] A medicinal composition containing velvet antler, which comprises by weight %: red deer antler freeze-dried powder 20%; glucosamine 10%; collagen 20%; vitamin B6 10%; dolomite 20%; germinated soybean powder 20% %.
[0037] All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com