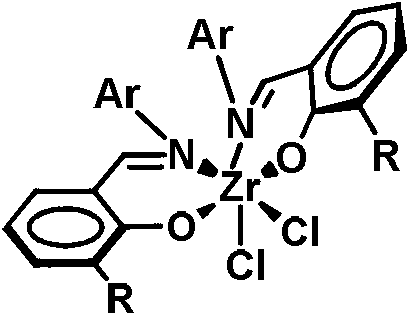
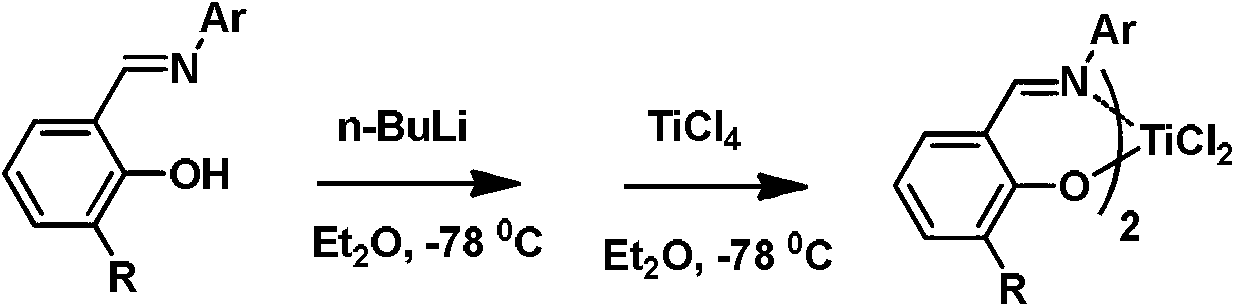
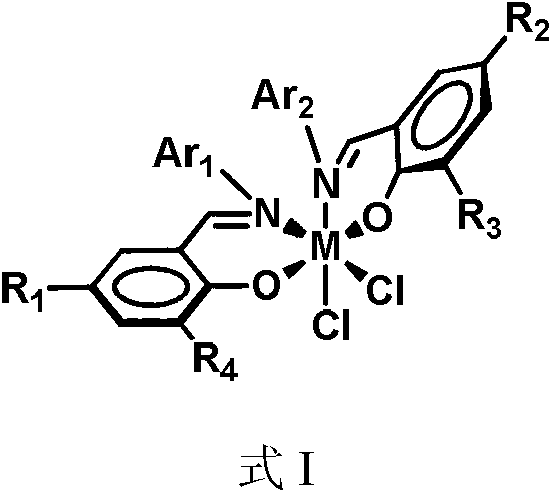
Metal miscellaneous ligand catalyst precursor based on salicylaldehyde imine ligand, as well as preparation and application thereof
A catalyst and precursor technology, applied in chemical instruments and methods, organic chemistry, titanium organic compounds, etc., can solve the problems of increasing the difficulty of catalyst synthesis and separation, and the inability to adjust catalyst activity. It is easy to achieve equivalent, simple and gentle operation, The effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of metal heteroligand catalyst precursor shown in formula IIa
[0043]
[0044] In a glove box, (E)-2-((pentafluorophenylimino)methyl)phenol (0.29 g, 1.00 mmol) was dissolved in anhydrous dichloromethane (DCM) solvent, An equivalent amount of potassium hydride was added to the solution and reacted for 1 hour. Afterwards, this solution is added dropwise to titanium metal monoligand complex (R in the compound shown in formula IV) at normal temperature 1 = H, R 4 =t-Bu, M=Ti) (0.56g, 1.00mmol) in dichloromethane solution, and reacted at this temperature for 3 hours. After the reaction was finished, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through celite, the filtrate was sucked dry, and the crude product was recrystallized with dichloromethane / n-hexane to obtain a reddish-brown powder (0.37g, 75%). 1 H NMR (CDCl 3 , 400MHz): δ8.30(s, 1H, CH=N), 8.18(s, 1H, CH=N), 7.70(d, 1H, J=7.2Hz, ArH), ...
Embodiment 2
[0046] The preparation of metal heteroligand catalyst precursor shown in formula IIb
[0047]
[0048] In the glove box, (E)-3-methyl-2-((pentafluorophenylimino)methyl)phenol (0.53g, 1.76mmol) was dissolved in anhydrous dichloromethane solvent, at room temperature Next, an equivalent amount of potassium hydride was added to the solution and reacted for 1 hour. Afterwards, this solution is added dropwise to titanium metal monoligand complex (R in the compound shown in formula IV) at normal temperature 1 = H, R 4 =t-Bu, M=Ti) (1.00 g, 1.76 mmol) in dichloromethane solution, and reacted at this temperature for 3 hours. After the reaction was finished, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through diatomaceous earth, the filtrate was sucked dry, and the crude product was recrystallized with dichloromethane / n-hexane to obtain a reddish-brown powder (0.50 g, 37%). 1 H NMR (CDCl 3 , 400MHz): δ8.29(s, 1H, CH=N), 8....
Embodiment 3
[0050] The preparation of metal heteroligand catalyst precursor shown in formula IIc
[0051]
[0052] In a glove box, (E)-3-((pentafluorophenylimino)methyl)-5-tert-butyl-[1,1'-biphenyl]-2-phenol (0.42 g, 1.00 mmol) was dissolved in anhydrous dichloromethane solvent, and an equivalent amount of potassium hydride was added to the solution at room temperature to react for 1 hour. Afterwards, this solution is added dropwise to titanium metal monoligand complex (R in the compound shown in formula IV) at normal temperature 1 =R 4 =t-Bu, M=Ti) (0.624g, 1.00mmol) in dichloromethane solution, and reacted at this temperature for 3 hours. After the reaction was finished, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through diatomaceous earth, the filtrate was sucked dry, and the crude product was recrystallized with dichloromethane / n-hexane to obtain a reddish-brown powder (0.43g, 46%). 1 H NMR (CDCl 3 , 400MHz): δ8.31(s, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com