A kind of preparation method of high molecular weight medicinal polyvinylpyrrolidone
The technology of polyvinylpyrrolidone and vinylpyrrolidone is applied in the fields of medicine, chemical industry and synthetic polymer, and can solve the problems such as difficulty in meeting pharmacopoeia requirements, difficulty in achieving uniform mixing, increase in energy consumption, etc. Guaranteed quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
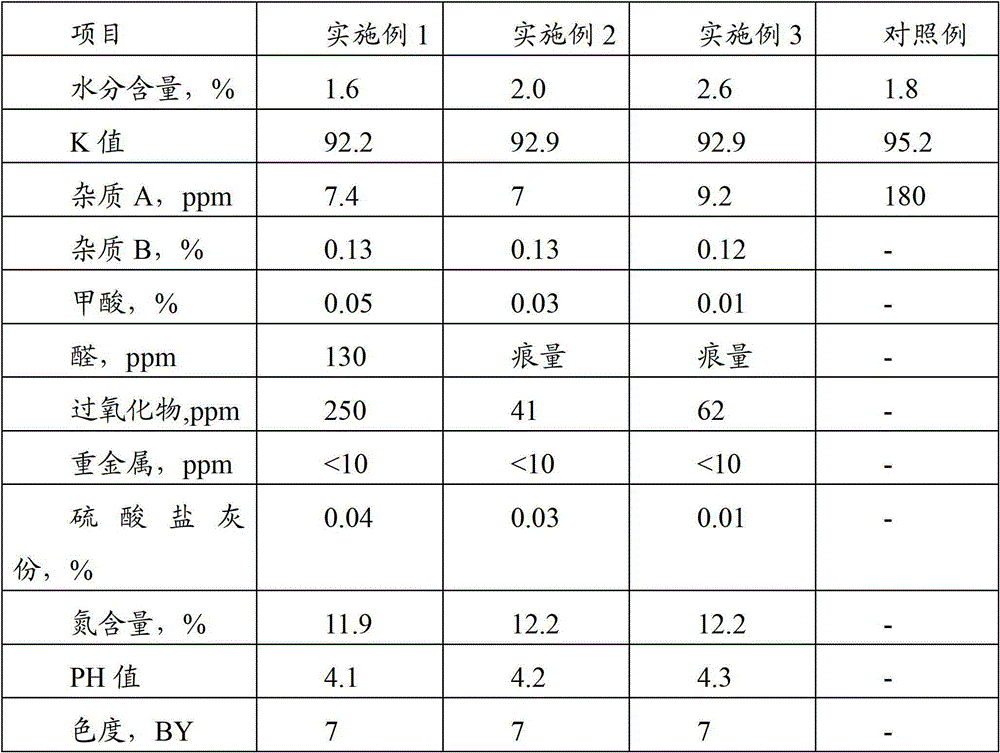
[0019] Put 3400kg of pure water into the reaction kettle equipped with heat exchange coil, nitrogen inlet pipe and stirrer, start the stirrer, introduce nitrogen and heat. After 20 minutes, 800kg of NVP was added, and when the material in the kettle was heated to 70°C, 1.0kg of azobisisobutyronitrile was added to initiate the polymerization reaction. Control material temperature fluctuations not greater than 2°C. After 5 hours, raise the temperature of the material to 75°C, and add 0.4 kg of a composite initiator of azobisisobutyronitrile and tert-butyl hydroperoxide mixed in a ratio of 1:0.3 every 4 hours, and throw in 4 times in total. After the last initiator was added, the reaction was maintained for another 8 hours, and the total polymerization time was 25 hours. The polymerization solution is filtered through a stainless steel mesh filter with a pore size of 1 μm, and then continuously enters a belt-type vacuum dryer, and is dried for 1.0 to 2.5 hours under the conditio...
Embodiment 2
[0021] 3400kg of pure water was dropped into the reactor described in Example 1, stirring was started, nitrogen was introduced and heated, and 800kg of NVP was dropped after 20 minutes. When the material was heated to 60°C, 1.5kg of a composite initiator of azobisisobutyronitrile and azobisisovaleronitrile mixed at a ratio of 1:1 was put in to initiate the polymerization reaction. Control the temperature fluctuation of the material to not exceed 2°C. After 6 hours, adjust the temperature of the material to 70°C. After that, add 0.5 kg of the above-mentioned composite initiator once every 5 hours, and throw 5 times in total. After the last input, the temperature was raised to 75° C., and the reaction was terminated after maintaining for 8 hours. The total polymerization time was 34 hours. After the polymerization solution was vacuum-dried and crushed at low temperature as described in Example 1, the obtained product was tested according to the Pharmacopoeia, and the data are s...
Embodiment 3
[0023] Put 3400kg of pure water into the reactor described in Example 1, start stirring, introduce nitrogen and heat, put in 800kg of NVP after 20 minutes, and when the material is heated to 65°C, put in azodiisocyanurate mixed in a ratio of 1:0.3 The composite initiator 1.2kg of butyronitrile and azobisisovaleronitrile initiates the polymerization reaction. Control the temperature fluctuation of the material not to exceed 2°C, adjust the temperature of the material to 75°C for 6 hours, add 0.4kg of the above-mentioned composite initiator, and then add 0.4kg each time every 4 hours, and throw 5 times in total. After the last input, the temperature was raised to 80° C., and the reaction was terminated after maintaining for 8 hours. The total polymerization time was 34 hours. After the polymerization solution was vacuum-dried and crushed at low temperature as described in Example 1, the obtained product was tested according to the Pharmacopoeia, and the data are shown in Table ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com