In-vitro detection method of allergen in food
A detection method and in vitro detection technology, applied in the direction of biological testing, material inspection products, etc., can solve the problem of inaccurate reflection of allergic reactions to allergens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
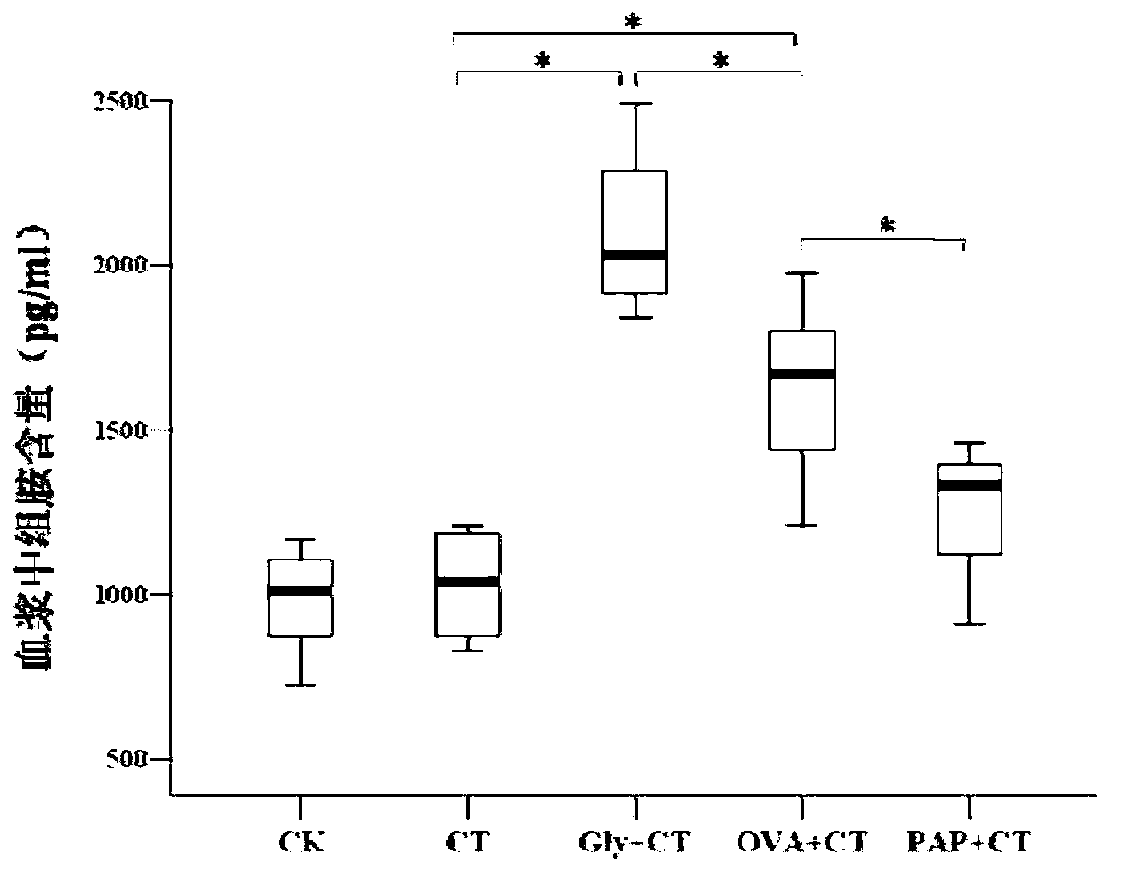
[0116] Embodiment 1 Animal experiment
[0117] 1. Experimental animals
[0118] 3-4 week old female BALB / c mice of SPF grade.
[0119] 2. Test substance
[0120] The tested proteins were glycinin (Glycinin, Gly), ovalbumin (OVA), potato acid phosphatase (PAP), all added with 10 μg cholera toxin adjuvant (CT, diluted in 100 μl normal saline)
[0121] 3. Animal grouping
[0122] The experimental animals were randomly divided into 5 groups according to body weight, namely: Gly+CT group, OVA+CT group, PAP+CT group, CT control group and negative control group.
[0123] 4. Dosage
[0124] The test protein was 1 mg, all adsorbed on 10 μg cholera toxin adjuvant (diluted in 100 μl normal saline), the CT control group received 10 μg cholera toxin adjuvant, and the negative control group received the same volume of normal saline.
[0125] 5. Animal handling
[0126] The experimental animals were subjected to the experiment after adaptive feeding for 1 week. Water and food were pro...
Embodiment 2
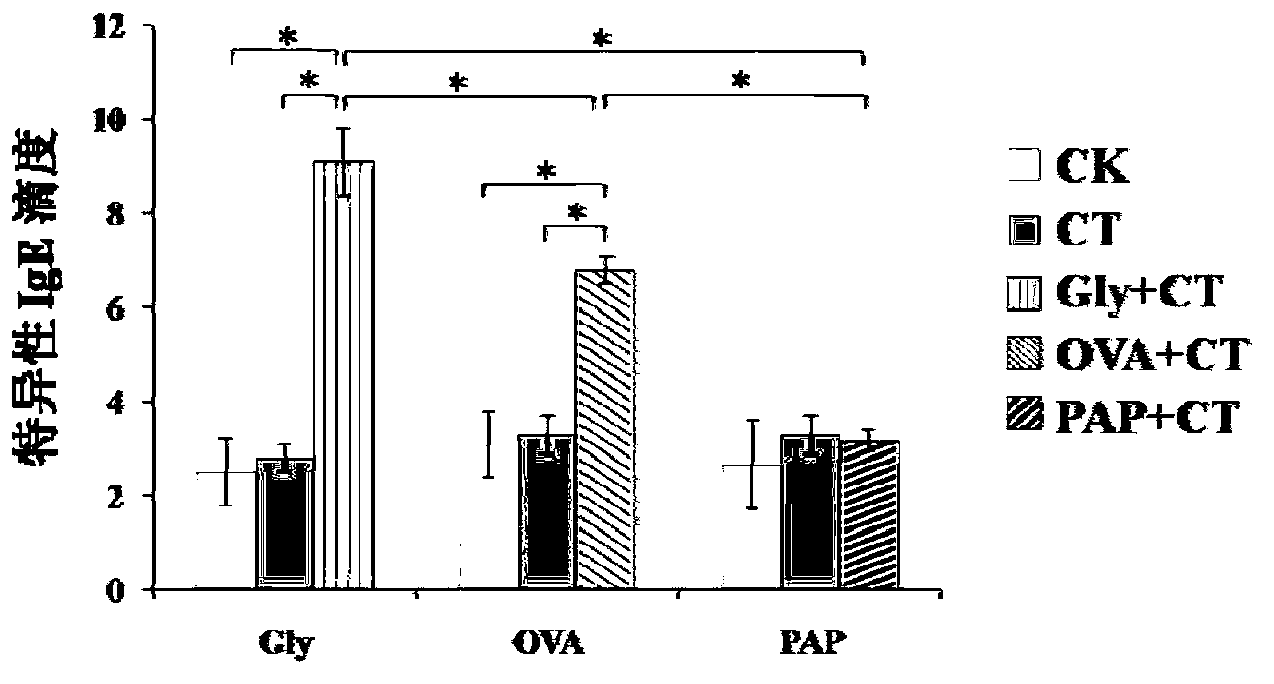
[0131] The detection of embodiment 2 specific IgE
[0132] 1) Coated
[0133] Dilute the protein to be tested to 10 mg / L with coating buffer, select wells to be coated on a 96-well plate, add 100 μl of coating solution to each well, and overnight at 4°C;
[0134] 2) washing
[0135] Pour off the liquid in the wells, add 250 μl of PBS / 0.1%BSA-Tween20 washing solution to each well, place at room temperature for 5 minutes, shake off the washing solution, pat several times on absorbent paper until there are no obvious drops in the wells, and repeat twice;
[0136] 3) closed
[0137] Add 150 μl PBS / 1%BSA to each well, incubate in a constant temperature incubator at 37°C for 1 hour, and wash three times;
[0138] 4) Add primary antibody
[0139] Add 100 μl of serum to be tested into each well, make three parallel holes for each test serum, and make three negative controls on each plate. Incubate in a constant temperature incubator at 37°C for 1 hour, and wash six times;
[014...
Embodiment 3
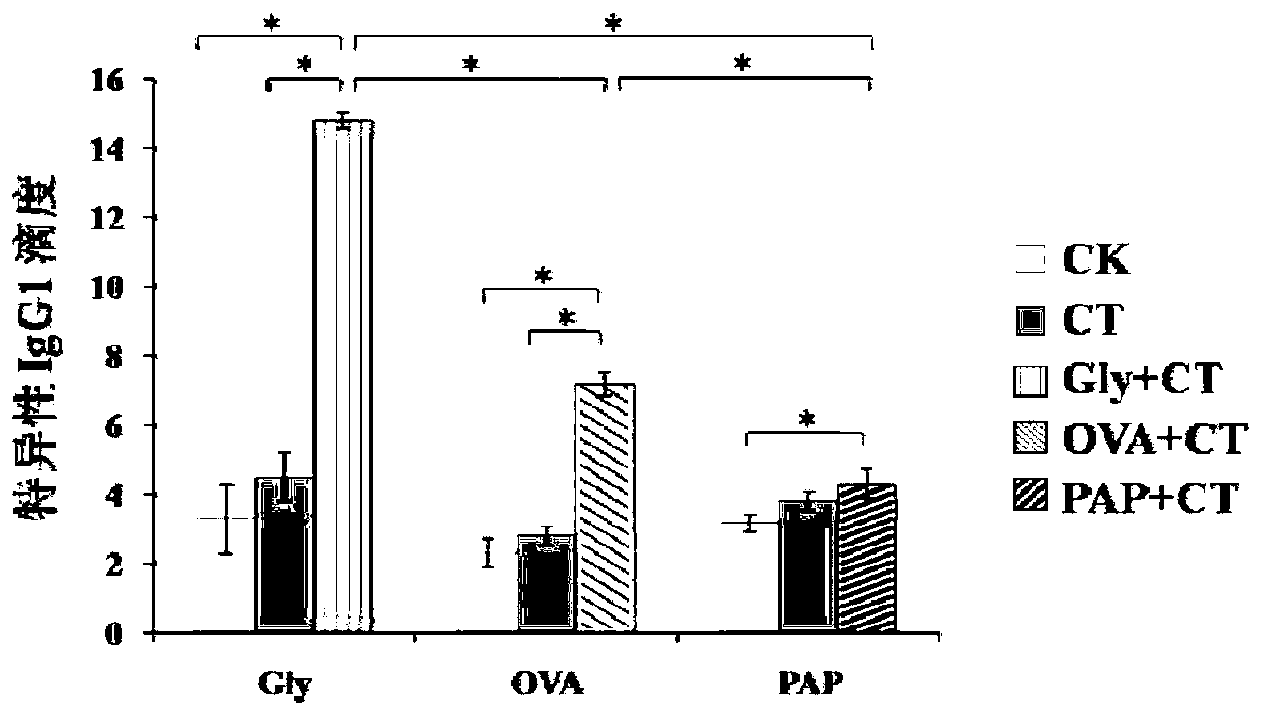
[0149] The detection of embodiment 3 specific IgG1
[0150] 1) Coated
[0151] Dilute the protein to be tested to 10 mg / L with coating buffer, select wells to be coated on a 96-well plate, add 100 μl of coating solution to each well, and overnight at 4°C;
[0152] 2) washing
[0153] Pour off the liquid in the wells, add 250 μl of PBS / 0.1%BSA-Tween20 washing solution to each well, place at room temperature for 5 minutes, shake off the washing solution, pat several times on absorbent paper until there are no obvious drops in the wells, and repeat twice;
[0154] 3) closed
[0155] Add 150 μl PBS / 1%BSA to each well, incubate in a constant temperature incubator at 37°C for 1 hour, and wash three times;
[0156] 4) Add primary antibody
[0157] Add 100 μl of serum to be tested into each well, make three parallel holes for each test serum, and make three negative controls on each plate. Incubate in a constant temperature incubator at 37°C for 1 hour, and wash six times;
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com