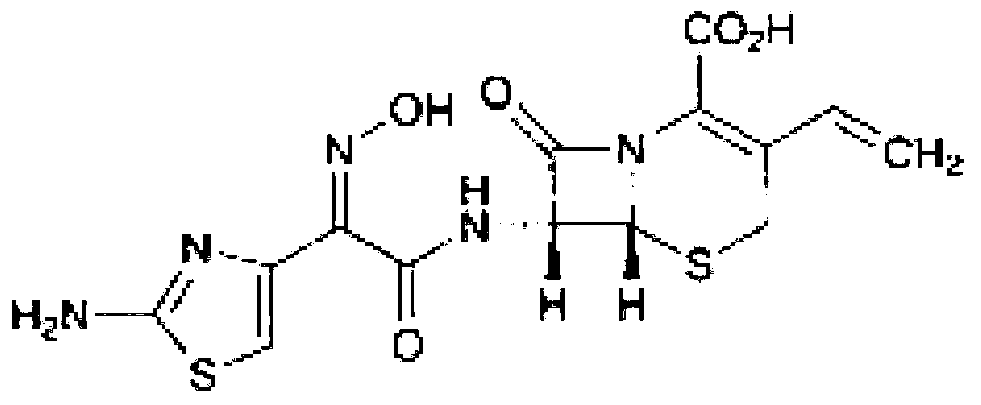
Cefdinir composition capsule and preparation method thereof
A technology for cefdinir and a composition, applied in the field of cefdinir composition capsules and preparation thereof, can solve the problems of low stability and low loss on drying, affecting the safety and effectiveness of cefdinir, inconvenience in production, and the like, and achieves satisfactory results. Beneficial for safe use and long-term storage, reducing or preventing digestive disorders, and improving stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
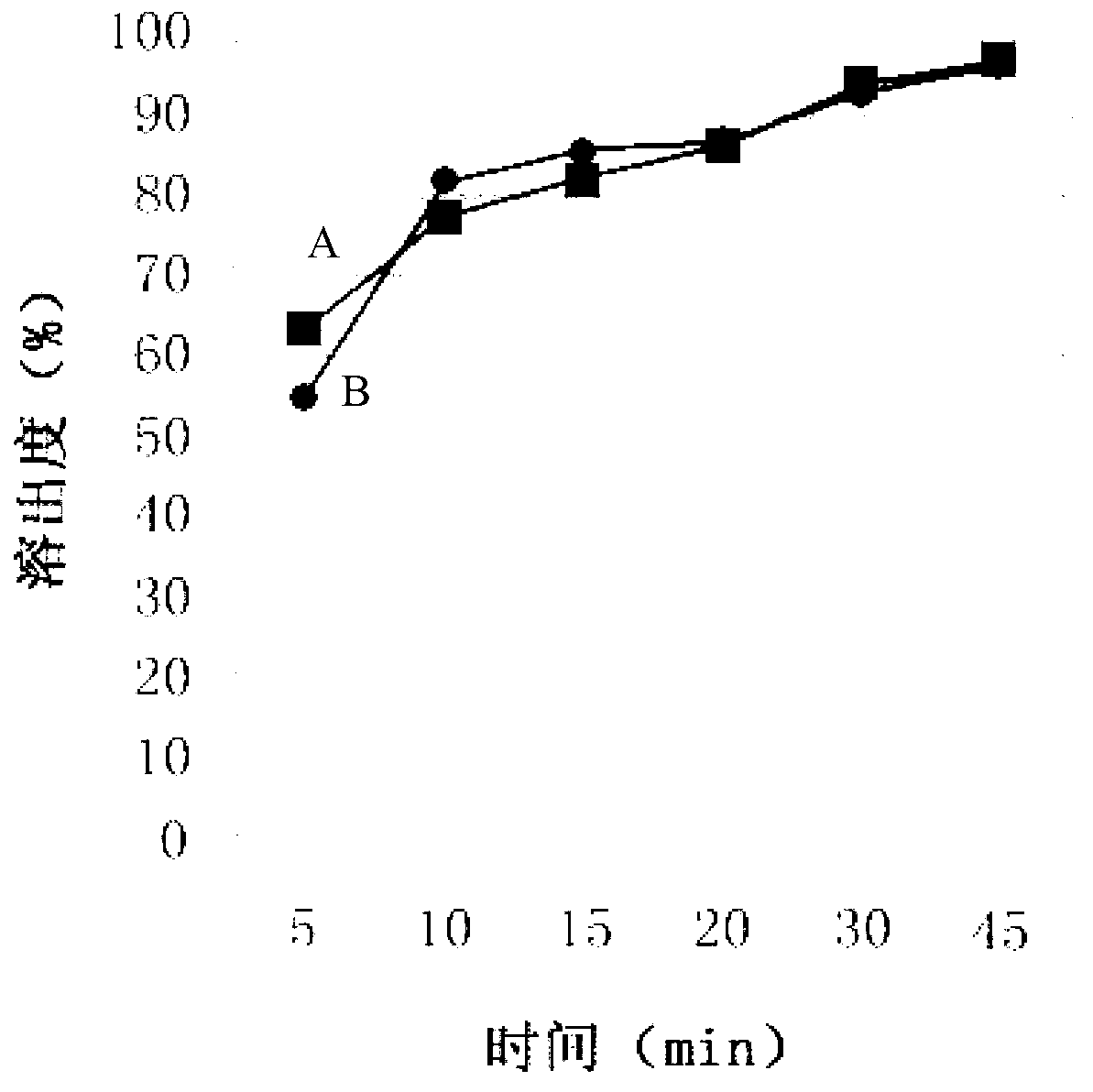
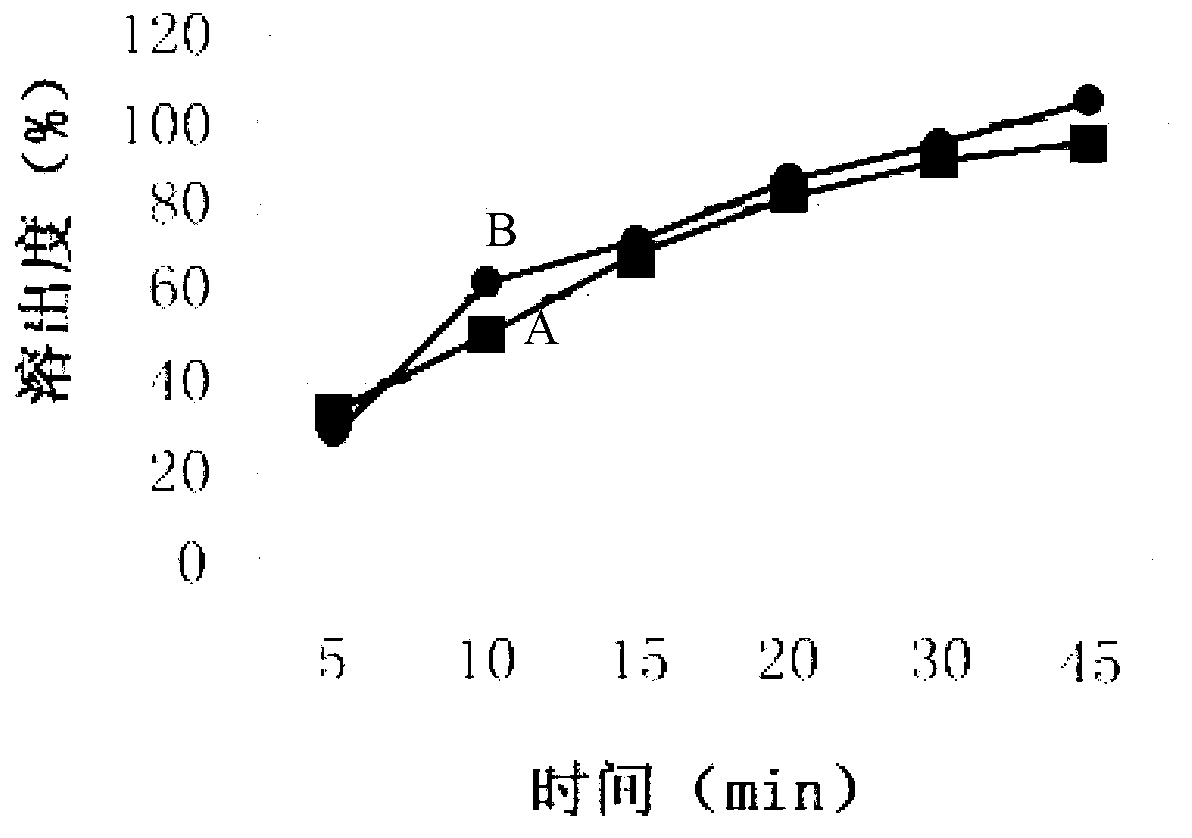
Image
Examples
Embodiment 1
[0027] Embodiment 1: prepare cefdinir composition capsule of the present invention
[0028]
[0029]
[0030] A total of 1000 capsules were made
[0031] Mix cefdinir through a 120 mesh sieve with polacrilin potassium and croscarmellose sodium that pass through a 100 mesh sieve respectively, and then mix them with microcrystalline cellulose, talcum powder and succinic acid that pass through a 100 mesh sieve respectively Mix evenly to get the mixture, add 95% ethanol solution and soft material made of 2-hydroxyethyl methacrylate to the mixture, wet granulate with 20 mesh sieve, blow dry the granules at about 65°C, granulate with 20 mesh sieve, Add magnesium stearate for total mixing, and fill No. 2 capsules.
Embodiment 2
[0032] Embodiment 2: preparation cefdinir composition capsule of the present invention
[0033]
[0034] A total of 1000 capsules were made
[0035] Mix cefdinir through a 120 mesh sieve with polacrilin potassium and croscarmellose sodium that pass through a 100 mesh sieve respectively, and then mix them with microcrystalline cellulose, talcum powder and succinic acid that pass through a 100 mesh sieve respectively Mix evenly to get the mixture, add 95% ethanol solution and soft material made of 2-hydroxyethyl methacrylate to the mixture, wet granulate with 20 mesh sieve, blow dry the granules at about 65°C, granulate with 20 mesh sieve, Add magnesium stearate for total mixing, and fill No. 2 capsules.
Embodiment 3
[0036] Embodiment 3: preparation cefdinir composition capsule of the present invention
[0037]
[0038]
[0039] A total of 1000 capsules were made
[0040]Mix cefdinir through a 120 mesh sieve with polacrilin potassium and croscarmellose sodium that pass through a 100 mesh sieve respectively, and then mix them with microcrystalline cellulose, talcum powder and succinic acid that pass through a 100 mesh sieve respectively Mix evenly to get the mixture, add 95% ethanol solution and soft material made of 2-hydroxyethyl methacrylate to the mixture, wet granulate with 20 mesh sieve, blow dry the granules at about 65°C, granulate with 20 mesh sieve, Add magnesium stearate for total mixing, and fill No. 2 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com