Aphanothece halophytica expolysaccharide immunoadjuvant
A technology of cryptobacterium algae and immune adjuvant, applied in the field of biomedicine, can solve the problems of inability to induce immunity, difficult standardization of quality standards, swelling, erythema, pain, induration and ecchymosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Hemolytic determination
[0014] In the experiment, rabbit heart was used to take blood, first inhale 3ml of sterile Ahrlid solution in the syringe, then take 7ml of blood from the heart, quickly push it into 4 times the volume of normal saline, shake well, centrifuge, and repeat the above operation with normal saline 3 The second highest clear was light yellow. Add 20ml of Aldrin's solution and mix and store at 4°C for later use.
[0015] The above red blood cells were diluted with normal saline into a 2% red blood cell suspension for experiment. The extracellular polysaccharide of Cryptococcum salina was diluted to 0.25, 0.50, 1.00 mg / ml with normal saline. Take 2ml of each and add 2ml 2% red blood cell suspension, mix well, and set the maximum and minimum hemolytic control with distilled water and normal saline respectively The above groups were placed in a constant temperature water bath at 37°C for 1 hour, the experimental phenomenon was observed, centrifuged, the sup...
Embodiment 2
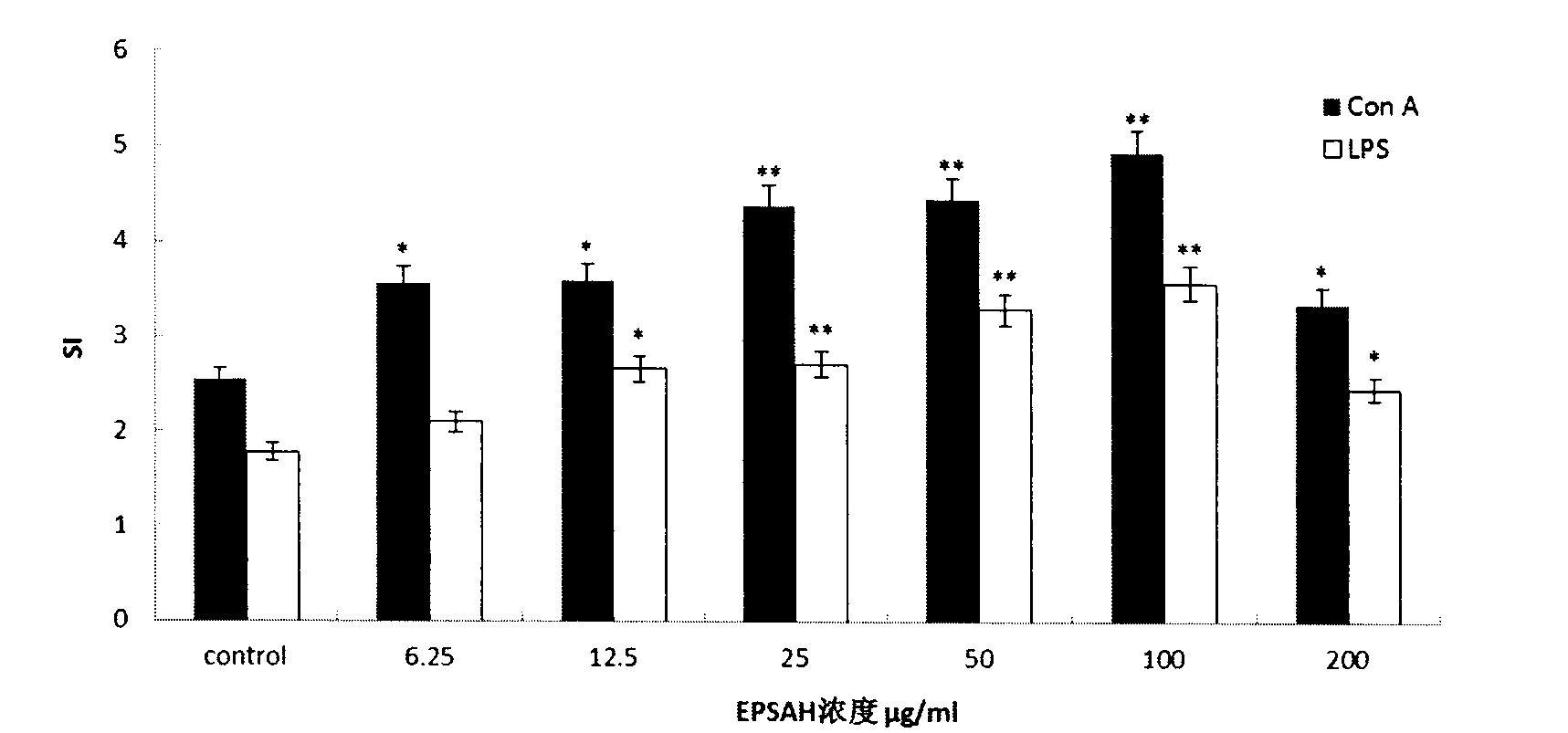
[0020] In vitro lymphocyte transformation experiment
[0021] Take fractional dislocations and put them to death. Take the spleen aseptically, add PBS solution, grind with a tissue homogenizer, and filter with a 100-mesh sieve to prepare a single cell suspension. After centrifugation, the supernatant was discarded, washed twice with PBS solution, and red blood cell lysate Tris-NH was added 4 Cl(NH 4 Cl 7.47g, Tris 2.60g, add ddH 2 0 to 1L, sterilized by 0.22 membrane filtration after autoclaving) 5ml, mix well and place at 37°C for 5min, add an equal volume of RPMI1640 complete medium (containing 10% FBS, 100μg / ml streptomycin, 100μg / ml penicillin) Discard the supernatant after centrifugation in the same way as above. Then wash twice with 1640 medium without serum, suspend the cells in 1640 complete medium, dilute with 10μl of spleen cell suspension, stain with trypan blue and count, the number of viable cells is not less than 95%; then prepare 5×10 6 A / ml mouse spleen lymphocyte...
Embodiment 3
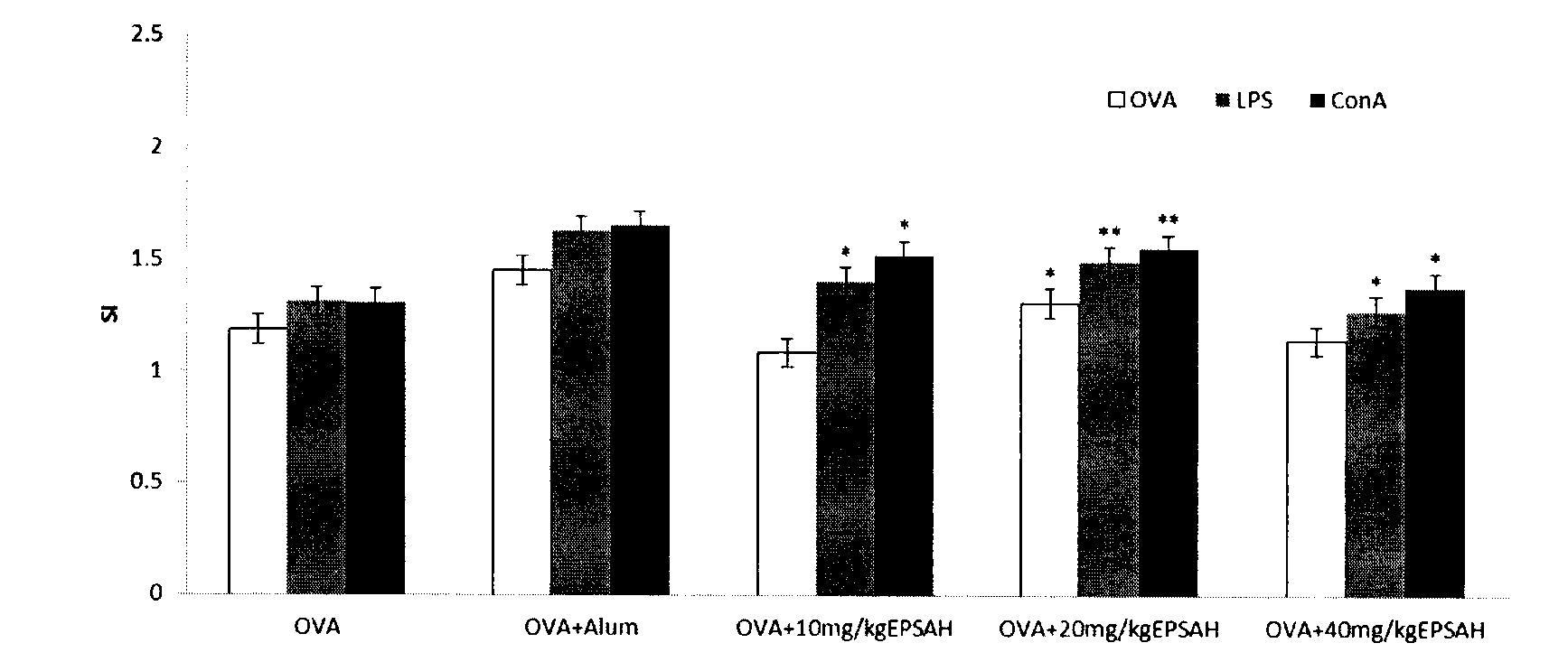
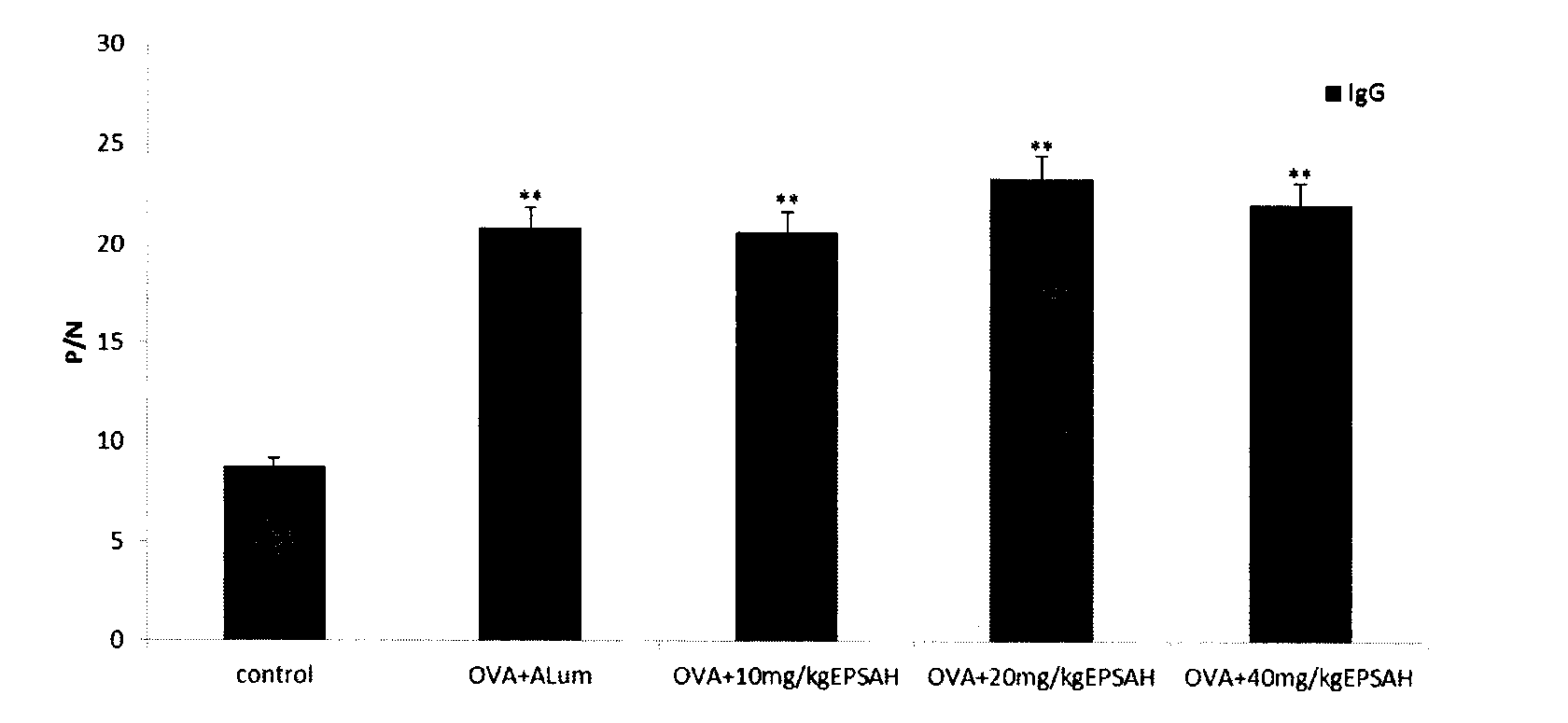
[0025] The effect of Cryptococcum salina exopolysaccharide on the cellular immunity of OVA-immunized mice
[0026] In this example, the physiological saline solution of C. salina exopolysaccharide and the physiological saline solution of OVA were uniformly mixed to obtain a mixed solution of C. salina exopolysaccharide and OVA; the physiological saline solution of aluminum gum adjuvant It is evenly mixed with the physiological saline solution of OVA to obtain a mixed solution of aluminum gel adjuvant and OVA.
[0027] 40 mice were randomly divided into groups, 8 mice in each group. Normal saline negative control group: each mouse was injected with 0.2ml normal saline; OVA control group: each mouse was injected with 0.2ml OVA physiological saline solution (0.5mg / ml); aluminum gel adjuvant (ALum) control group: each Mice were injected with a mixture of 0.1ml OVA physiological saline solution (1mg / ml) and 0.1ml aluminum gel adjuvant (ALum) physiological saline solution (2mg / ml); Cryp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com