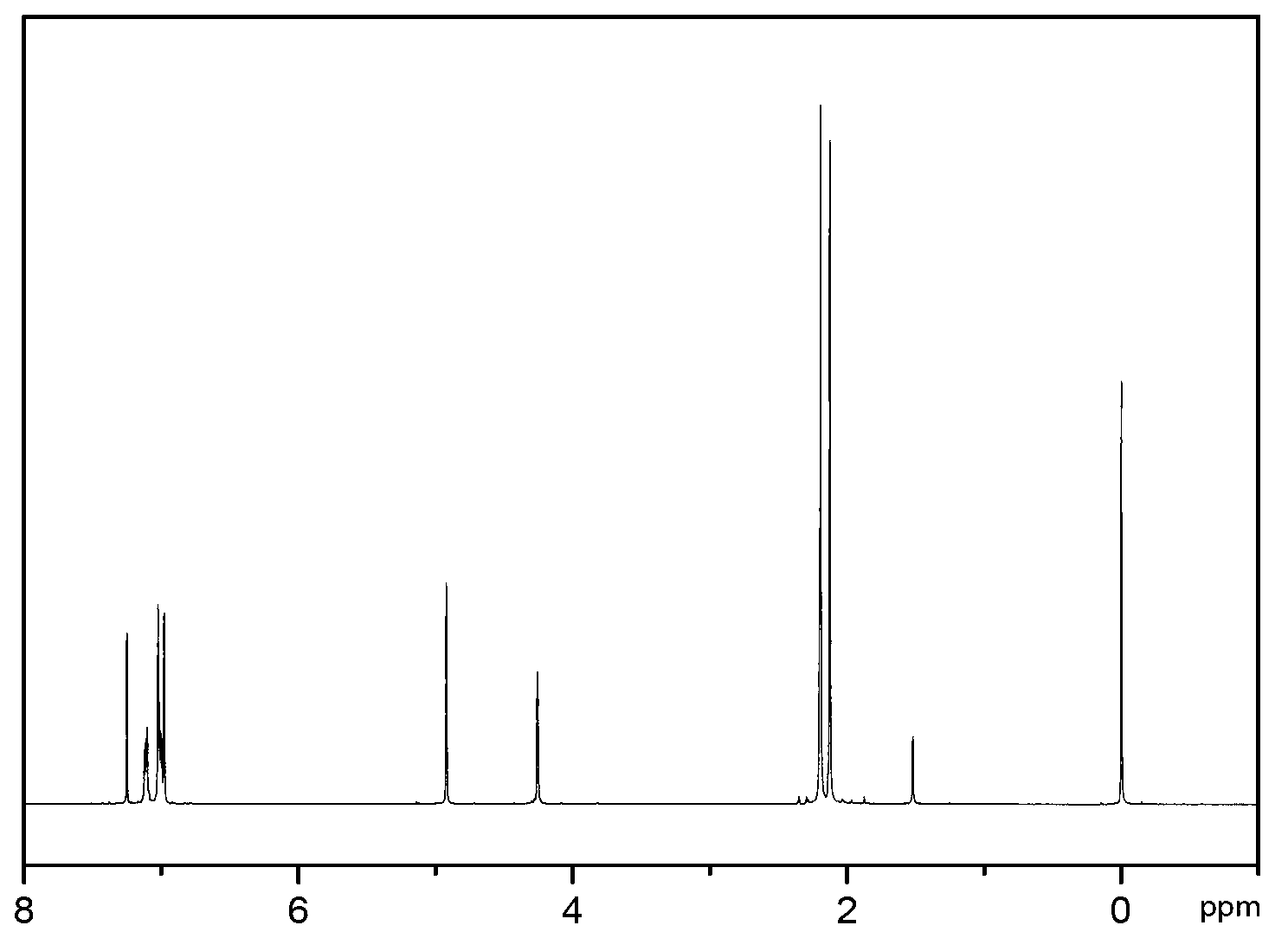
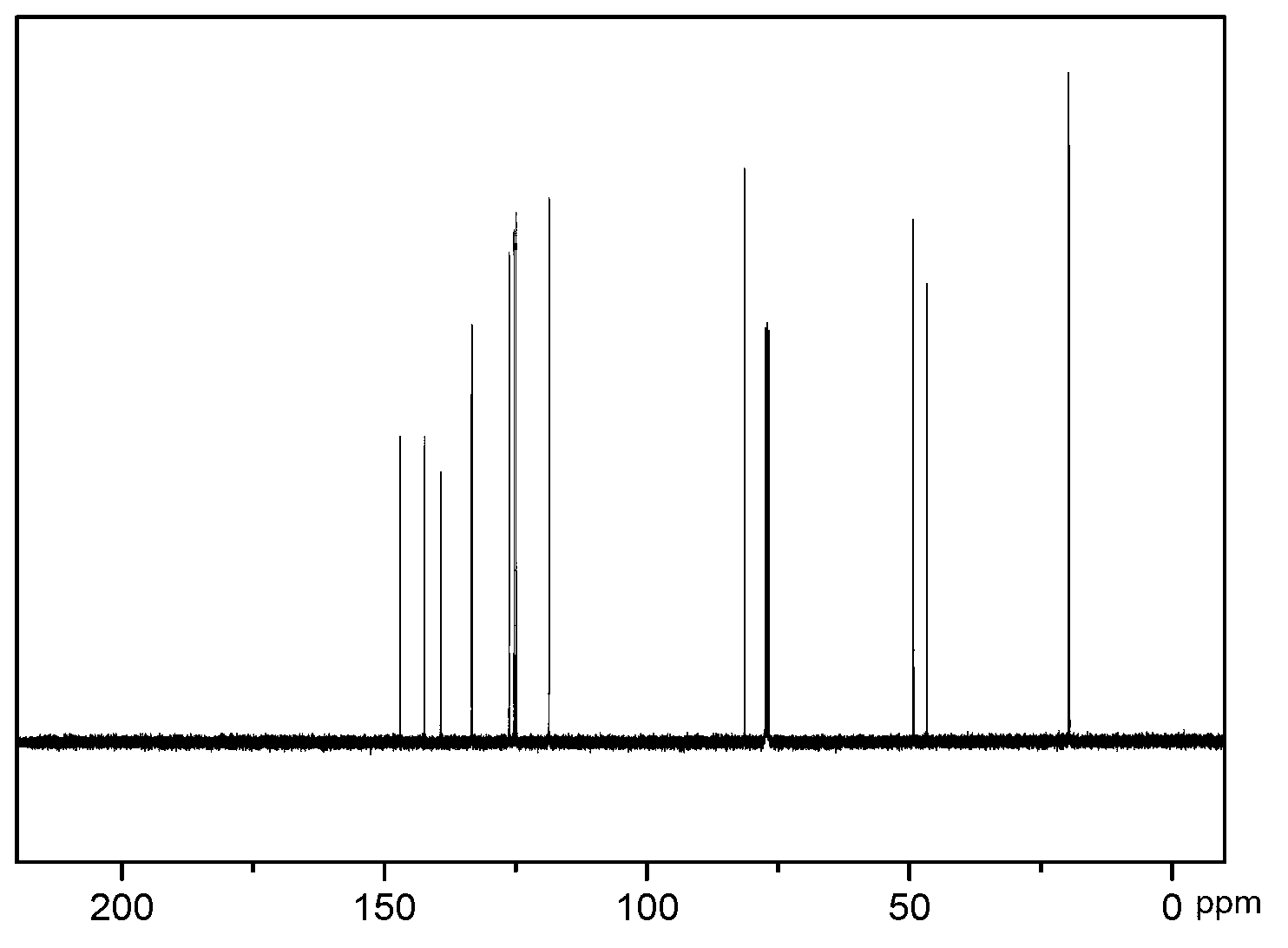
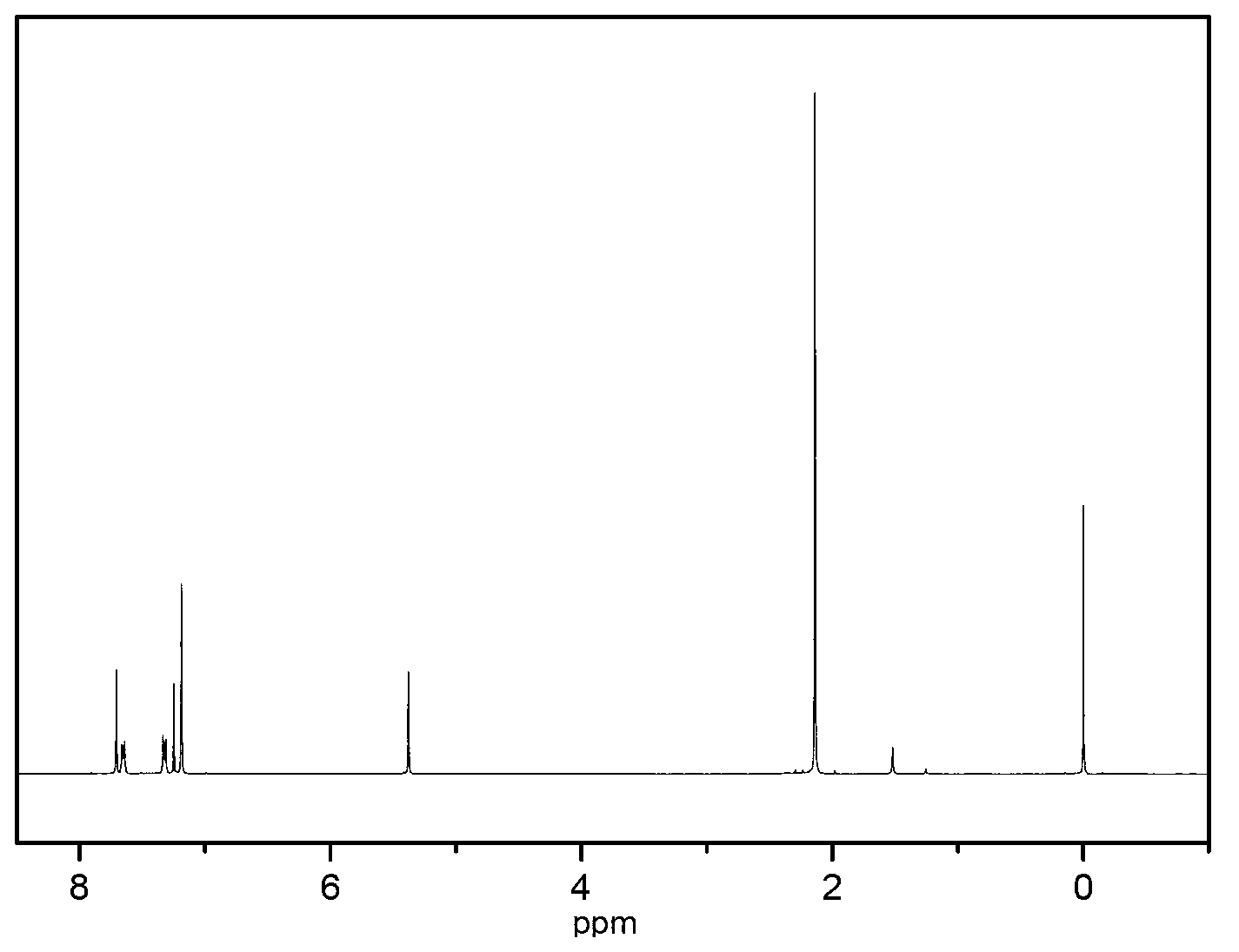
2, 3, 6, 7-tetramethyl-9,10-o-naphthylanthracene and synthetic method thereof
A technology of o-naphthyl anthracene and synthesis method, applied in 2 fields, can solve the problems of few dianhydride monomers, complicated synthesis, etc., and achieve the effects of good solubility and stability, simple preparation, high synthesis yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also relates to a synthesis method of 2,3,6,7-tetramethyl-9,10-o-naphthyl anthracene, comprising the following steps:
[0037] Step 10, o-xylene and dichloromethane react under the catalysis of aluminum trichloride, and generate 2,3,6,7-tetramethylanthracene by heating up through Friedel-Crafts reaction;
[0038] Step 20, 2,3,6,7-tetramethylanthracene and 1,4-epoxy-1,4-dihydronaphthalene are heated to reflux in an organic solvent to undergo a Diels-Alder reaction to generate 2,3,6,7- Tetramethyl-9,10-o-(1,4-epoxynaphthyl)anthracene crude product;
[0039] Step 30. The crude 2,3,6,7-tetramethyl-9,10-o-(1,4-epoxynaphthyl)anthracene is separated by column chromatography and washed with a mixed eluent to obtain 2,3,6, 7-Tetramethyl-9,10-o-(1,4-epoxynaphthyl)anthracene;
[0040] Step 40. 2,3,6,7-tetramethyl-9,10-o-(1,4-epoxynaphthyl)anthracene is dehydrated under acetic acid / acetic anhydride under reflux to obtain 2,3,6,7 -Tetramethyl-9,10-o-naphthyl...
Embodiment 1
[0051] The synthesis method of 2,3,6,7-tetramethyl-9,10-o-naphthylanthracene in this embodiment is as follows: Measure 120ml o-xylene and 65ml dichloromethane and mix together, and cool in an ice bath To 0-5°C, add solid aluminum trichloride in batches under vigorous stirring. After the addition, react at 0-5°C for 1 hour, then rise to room temperature for 1 hour, and then react at 60°C for 5 hours. After the reaction, the above reaction solution was poured into glacial hydrochloric acid aqueous solution, stirred, static, filtered and recrystallized with toluene to obtain 22g of white to yellow needle-like crystals of 2,3,6,7-tetramethylanthracene, melting point 299°C.
[0052] 6.50g (27.8mmol) of 2,3,6,7-tetramethylanthracene and 2.00g (13.9mmol) of 1,4-epoxy-1,4-dihydronaphthalene were added to a 250ml three-necked flask, and Add 100ml of xylene, then heat to reflux, monitor the end of the reaction with TLC, and reflux for 120h. After the reaction, the reaction bottle was c...
Embodiment 2
[0069] The synthesis method of 2,3,6,7-tetramethyl-9,10-o-naphthylanthracene of the present embodiment is as follows: Measure 120ml o-xylene and 75ml dichloromethane and mix together, and cool in an ice bath To 0-5°C, add solid aluminum trichloride in batches under vigorous stirring. After the addition, react at 0-5°C for 1 hour, then rise to room temperature for 1 hour, and then react at 70°C for 3 hours. After the reaction, the above reaction solution was poured into glacial hydrochloric acid aqueous solution, stirred, static, filtered and recrystallized with toluene to obtain 21 g of white to yellow needle-like crystals of 2,3,6,7-tetramethylanthracene, melting point 299°C.
[0070] 6.50g (27.8mmol) of 2,3,6,7-tetramethylanthracene and 2.00g (13.9mmol) of 1,4-epoxy-1,4-dihydronaphthalene were added to a 250ml three-necked flask, and Add 100ml of toluene, then heat to reflux, monitor the end of the reaction with TLC, and reflux for 120h. After the reaction, the reaction bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com