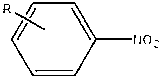
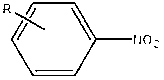
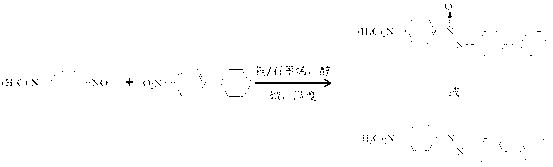Method for photocatalytic synthesis of azoxybenzene and azobenzene compounds
A technology for oxidizing azobenzene and compounds, applied in the direction of organic chemistry, etc., can solve the problems of unsuitable industrial catalysts, expensive gold, etc., and achieve the effects of high product yield, high selectivity and short reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] After mixing nitrobenzene, potassium hydroxide and ethanol solution uniformly according to the molar ratio of 1:0.12:200, add a copper / graphene catalyst with a copper content of 2wt% according to the mass ratio of 1:0.001, and disperse it by ultrasonic (electric power 60W) for 30 minutes , the dispersed suspension was heated to 40°C under the protection of xenon light and argon o C stirring and reacting for 6h can obtain azobenzene oxide, wherein the conversion rate of nitrobenzene is 90%, and the selectivity of azobenzene oxide is 94%; heating to 100 o C Stirring reaction 5h reaction can obtain azobenzene, wherein the conversion rate of nitrobenzene is 95%, and the selectivity of azobenzene is 98%.
Embodiment 2
[0034]
[0035] After mixing p-chloronitrobenzene, potassium tert-butoxide and 2-heptanol solution according to the molar ratio of 1:0.3:500, add a copper / graphene catalyst with a copper content of 7wt.% according to the mass ratio of 1:0.01, and ultrasonically (Electric power 80W) After dispersing for 90 minutes, heat the dispersed suspension to 80°C under the protection of xenon light and neon gas. o C stirred and reacted for 3h to obtain p-azobenzene oxychloride, wherein the conversion rate of p-chloronitrobenzene was 91%, and the selectivity of p-azobenzene oxychloride was 89%; heating to 100 o C Stirred and reacted for 3 hours to obtain p-chloroazobenzene, wherein the conversion rate of p-chloronitrobenzene was 94%, and the selectivity of p-chloroazobenzene was 85%.
Embodiment 3
[0037]
[0038] After p-methylnitrobenzene, sodium tert-butoxide and isopropanol solution are mixed uniformly according to the molar ratio 1:0.24:240, add the copper / graphene catalyst that copper content is 5wt% by mass ratio 1:0.04, ultrasonic ( Electric power 150W) After dispersing for 30min, the dispersed suspension was heated to 60°C under the protection of xenon light and nitrogen o C stirring reaction 5h can obtain p-methylazobenzene oxide, wherein p-methylnitrobenzene conversion rate is 91%, and p-methylazobenzene oxide selectivity is 90%; Heating to 110 o C Stirred reaction 5h reaction can obtain p-methylazobenzene, wherein the conversion rate of p-methylnitrobenzene is 95%, and the selectivity of p-methylazobenzene is 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com