Method for preparing pharmaceutical dextran and levulose by bi-enzyme coupled multistage membrane separation
A technology for separation of dextran and membrane, applied in the direction of fructose production, sugar production, application, etc., can solve the problems of complicated separation of follow-up products, low yield of target products, and affecting the safe use of medicinal dextran, so as to facilitate industrial promotion and application , high added value, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
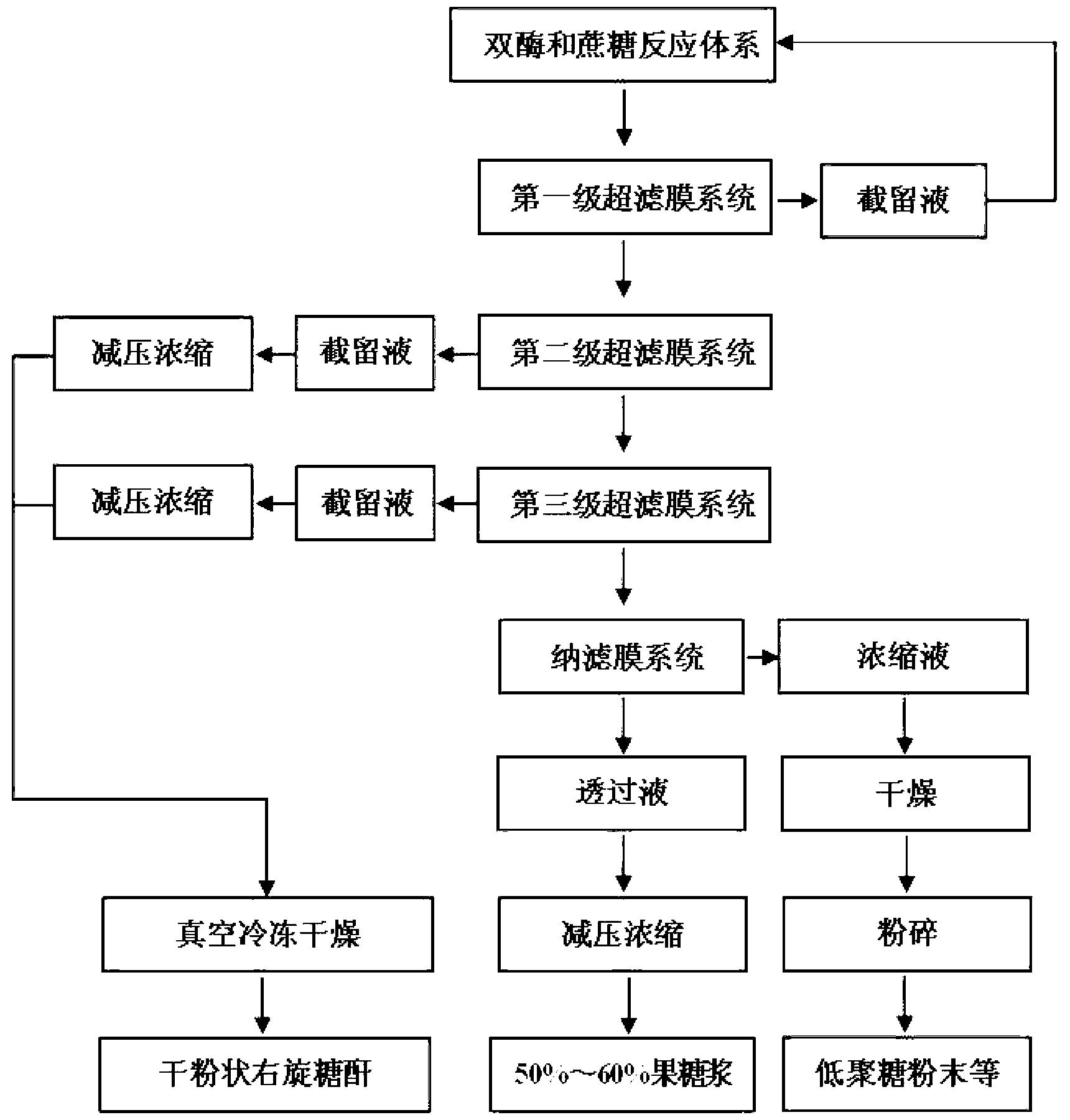
[0022] Dextransucrase was fermented by Leuconostoc enterococci, the fermentation broth was centrifuged at 12,000r / min and 4°C for 20min, and the supernatant was obtained. The enzyme activity was detected by DNS method. Dextranase comes from Amano Amano Enzyme Trading (Shanghai) Co., Ltd. According to the enzyme activity assay method provided by Amano and combined with the actual situation of the present invention, the assay conditions were slightly modified to measure the enzyme activity of dextranase. It was determined that the enzyme activity of dextran sucrase was 5 U / mL, and the enzyme activity of dextranase was 22,000 U / mL.
[0023]Use sterile water to prepare 1M sucrose solution as the substrate mother solution, and then the final concentration of the substrate is 0.6M, and the final concentration of the enzyme is 0.5U / mL (dextran sucrase) and 0.05U / mL (dextranase). Add the two kinds of enzyme liquids (i.e. keep the ratio of the enzyme activity of dextran sucrase and dex...
Embodiment 2
[0025] The acquisition of dextran sucrase and dextranase and the determination of enzyme activity are the same as in Example 1.
[0026] Use sterile water to prepare 1M sucrose solution as the substrate mother solution, and then the final concentration of the substrate is 0.4M, and the final concentration of the enzyme is 0.5U / mL (dextran sucrase) and 0.05U / mL (dextranase). Add the two kinds of enzyme liquids (i.e. keep the ratio of the enzyme activity of dextran sucrase and dextranase in the reaction system at 10:1), the preparation of the reaction liquid is diluted with sterile water, and 4L of the reaction liquid is prepared at one time. The reaction temperature was 24°C and the pH was 6.3. The reaction liquid is placed in the barrel at the front end of the membrane separation, and the first-stage ultrafiltration membrane module with a molecular weight cut-off of 50,000 is started, and the outlet pressure is adjusted to 0.03-0.15MPa (about 5-15psi, or the membrane Separati...
Embodiment 3
[0028] The acquisition of dextran sucrase and dextranase and the determination of enzyme activity are the same as in Example 1.
[0029] Use sterile water to prepare 1M sucrose solution as the substrate mother solution, and then the final concentration of the substrate is 0.2M, and the final concentration of the enzyme is 0.5U / mL (dextran sucrase) and 0.05U / mL (dextranase). Add the two kinds of enzyme liquids (i.e. keep the ratio of the enzyme activity of dextran sucrase and dextranase in the reaction system at 10:1), the preparation of the reaction liquid is diluted with sterile water, and 4L of the reaction liquid is prepared at one time. The reaction temperature was 25°C and the pH was 6.5. The reaction liquid is placed in the barrel at the front end of the membrane separation, and the first-stage ultrafiltration membrane module with a molecular weight cut-off of 70,000 is started, and the outlet pressure is adjusted to 0.03-0.15MPa (about 5-15psi, or the membrane Separati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com