Injection-use pharmaceutical composition comprising cefathiamidine and tazobactam sodium
A technology of tazobactam sodium and cefathiamidine, which is applied to the active ingredients of heterocyclic compounds, antibacterial drugs, and pharmaceutical formulations, and can solve the problems of large influence on antibacterial activity, differences in inhibitory enzyme spectrum and stability, and antibacterial activity The degree of optimization cannot be accurately predicted, and achieve the effect of high antibacterial activity, excellent stability, and a wide range of storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
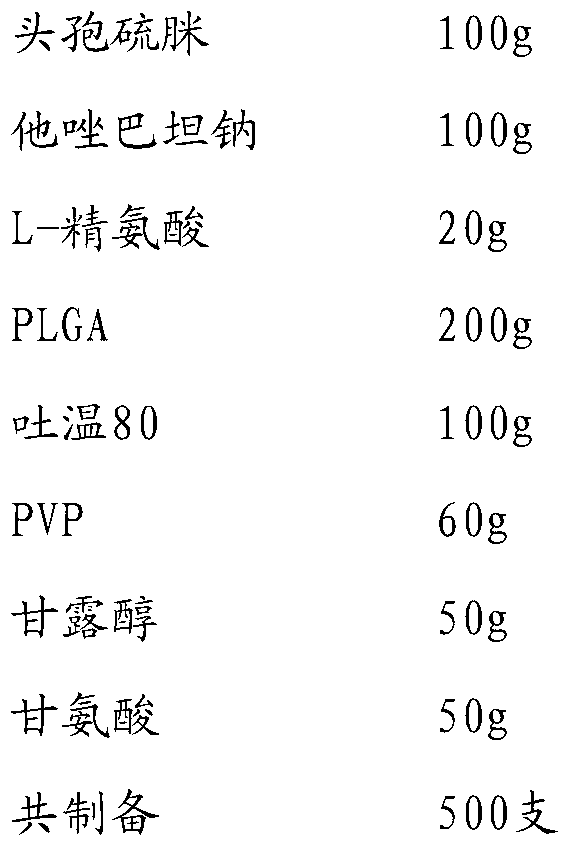
[0029]
[0030] Add cefathiamidine, L-arginine and tazobactam sodium to 800ml water for injection in sequence, stir and dissolve to obtain the inner water phase W1; mix PLGA (molecular weight about 6000, LA:GA polymerization ratio 50:50), Tween Dissolve in 1200 methylene chloride / acetone with a volume ratio of 3:1 to obtain the oil phase; add PVP, mannitol and glycine to 800 water for injection and stir until completely dissolved to obtain the external water phase W2; slowly add W1 under stirring In the oil phase, ultrasonically treat for 20s under ice bath to obtain colostrum; slowly add colostrum into W2 and stir for 10 minutes to obtain double emulsion, pour the double emulsion into 1500ml of sodium chloride aqueous solution for injection, stir in ice bath for 4 hours to evaporate residual organic solvent , the microspheres were collected by filtration through a 0.45 μm microporous membrane, washed three times with water for injection, and vacuum freeze-dried.
Embodiment 2
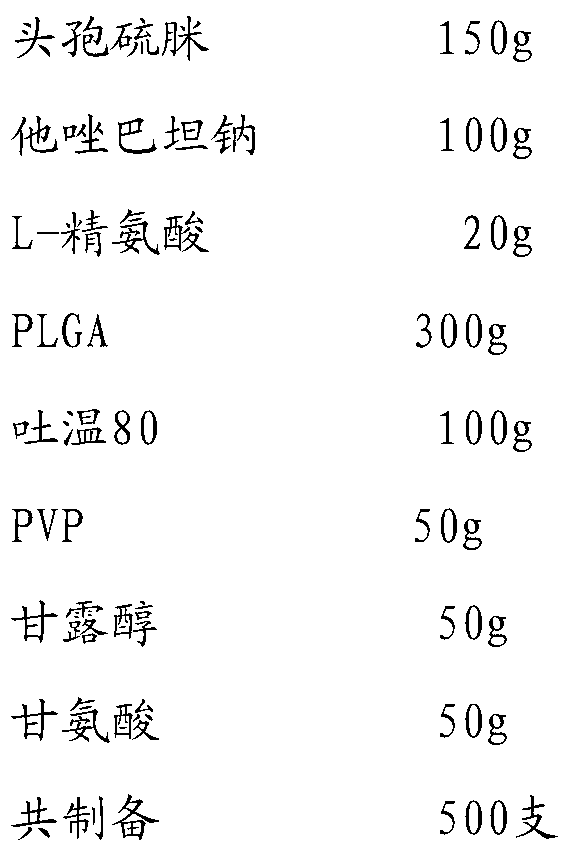
[0032]
[0033] Add cefathiamidine, L-arginine and tazobactam sodium to 800ml water for injection in turn, stir and dissolve to obtain the inner water phase W1; mix PLGA (molecular weight about 5500, LA:GA polymerization ratio 50:50), Tween Dissolve in 1200ml of dichloromethane / acetone with a volume ratio of 3:1 to obtain the oil phase; add PVP, mannitol and glycine to 800ml of water for injection and stir until completely dissolved to obtain the external water phase W2; slowly add W1 under stirring In the oil phase, ultrasonically treat for 20s under ice bath to obtain colostrum; slowly add colostrum into W2 and stir for 10 minutes to obtain double emulsion, pour the double emulsion into 1500ml of sodium chloride aqueous solution for injection, stir in ice bath for 4 hours to evaporate residual organic solvent , the microspheres were collected by filtration through a 0.45 μm microporous membrane, washed three times with water for injection, and vacuum freeze-dried.
Embodiment 3
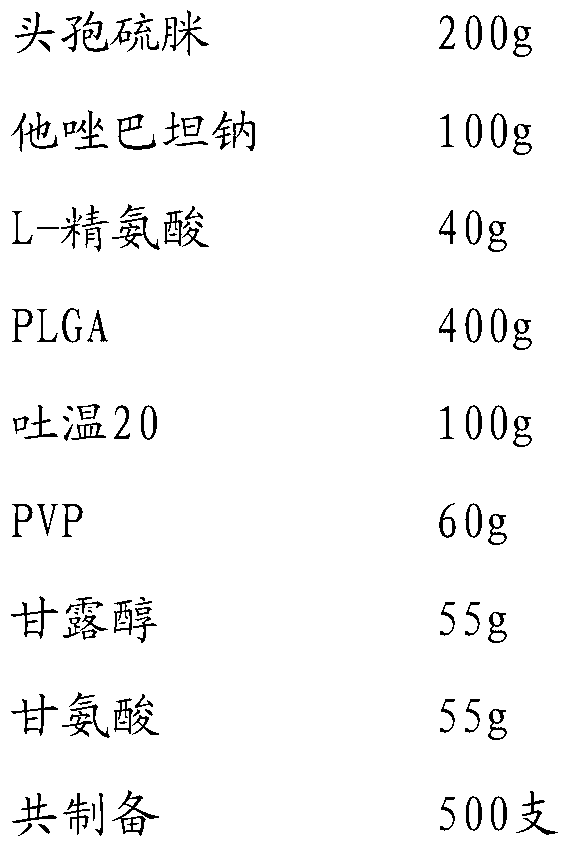
[0035]
[0036] Add cefathiamidine, L-arginine and tazobactam sodium to 1000% water for injection in turn, stir and dissolve to obtain the inner water phase W1; PLGA (molecular weight is about 5000, LA:GA polymerization ratio 50:50), Tween Dissolve in 1200ml of dichloromethane / acetone with a volume ratio of 3:1 to obtain the oil phase; add PVP, mannitol and glycine to 800ml of water for injection and stir until completely dissolved to obtain the external water phase W2; slowly add W1 under stirring In the oil phase, ultrasonically treat for 20s under ice bath to obtain colostrum; slowly add colostrum into W2 and stir for 10 minutes to obtain double emulsion, pour the double emulsion into 1500ml of sodium chloride aqueous solution for injection, stir in ice bath for 4 hours to evaporate residual organic solvent , the microspheres were collected by filtration through a 0.45 μm microporous membrane, washed three times with water for injection, and vacuum freeze-dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com