Polylactide containing side hydroxy or side carboxy functional group and preparation method thereof
A technology of polylactide and pendant carboxyl groups, which is applied in the field of polylactide and its preparation, can solve the problems of lack of functional groups, poor water solubility, and single chemical structure, and achieve the effects of good hydrophilicity, low cost, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of polylactide containing pendant monohydroxy functional groups:
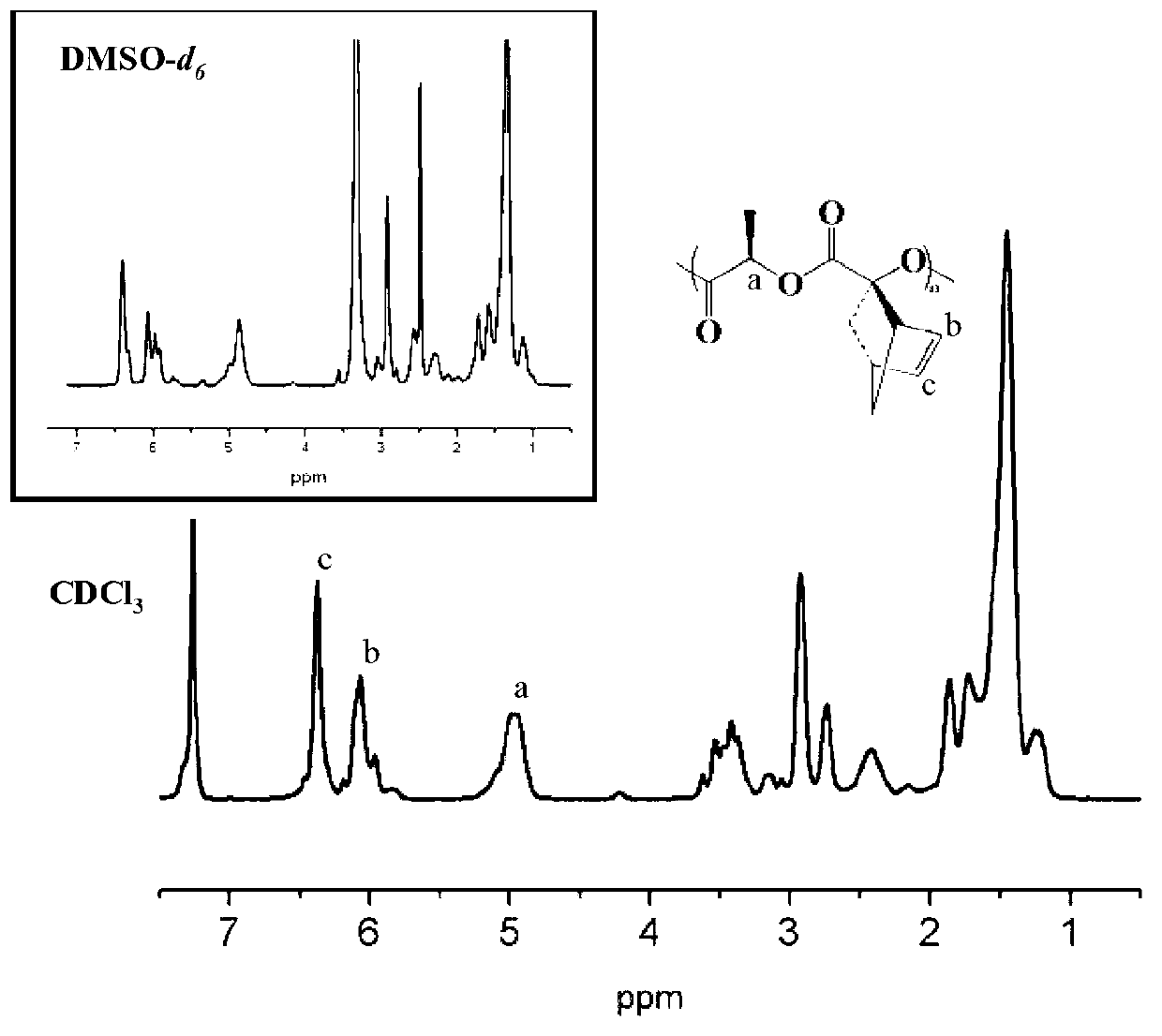
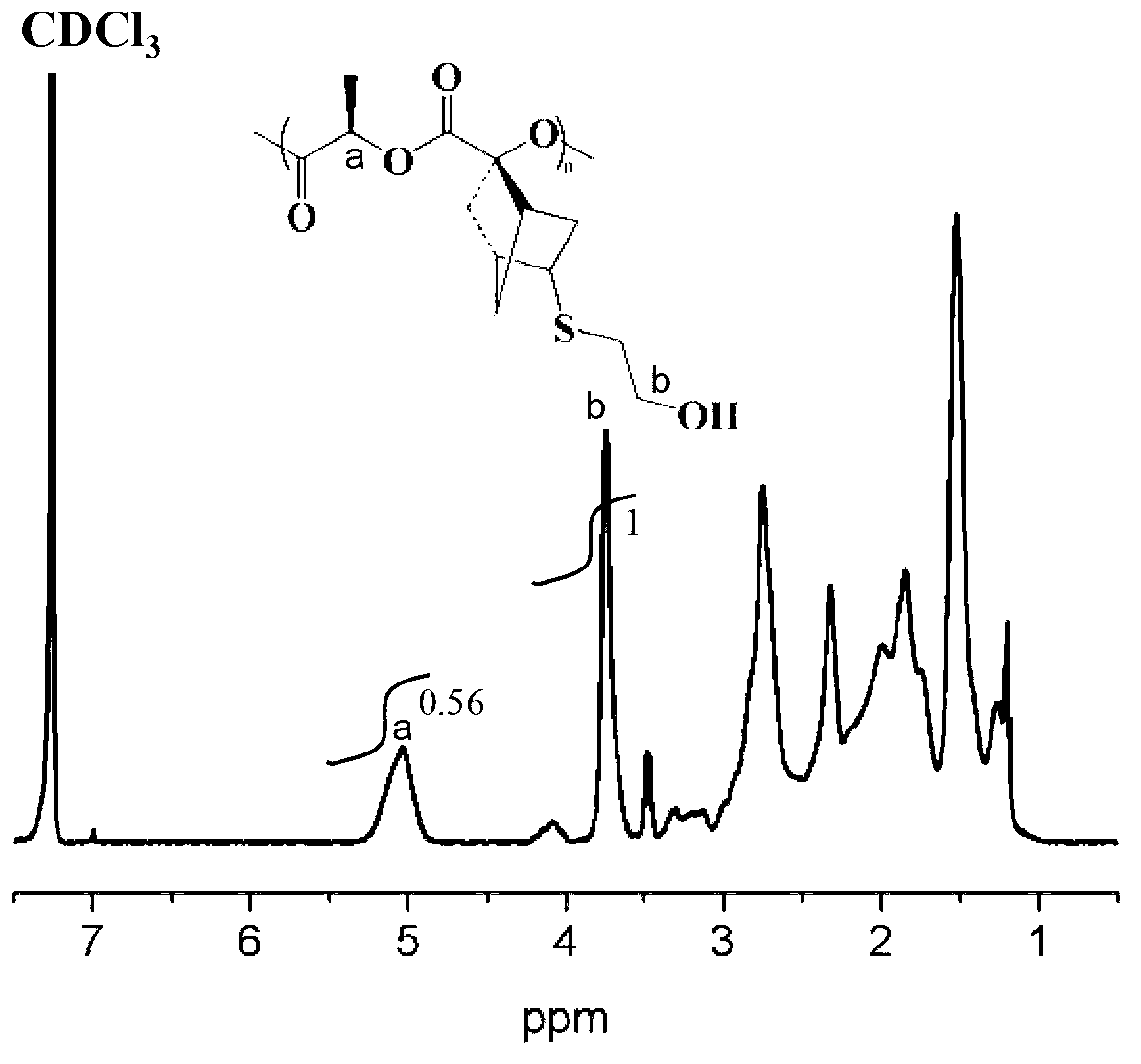
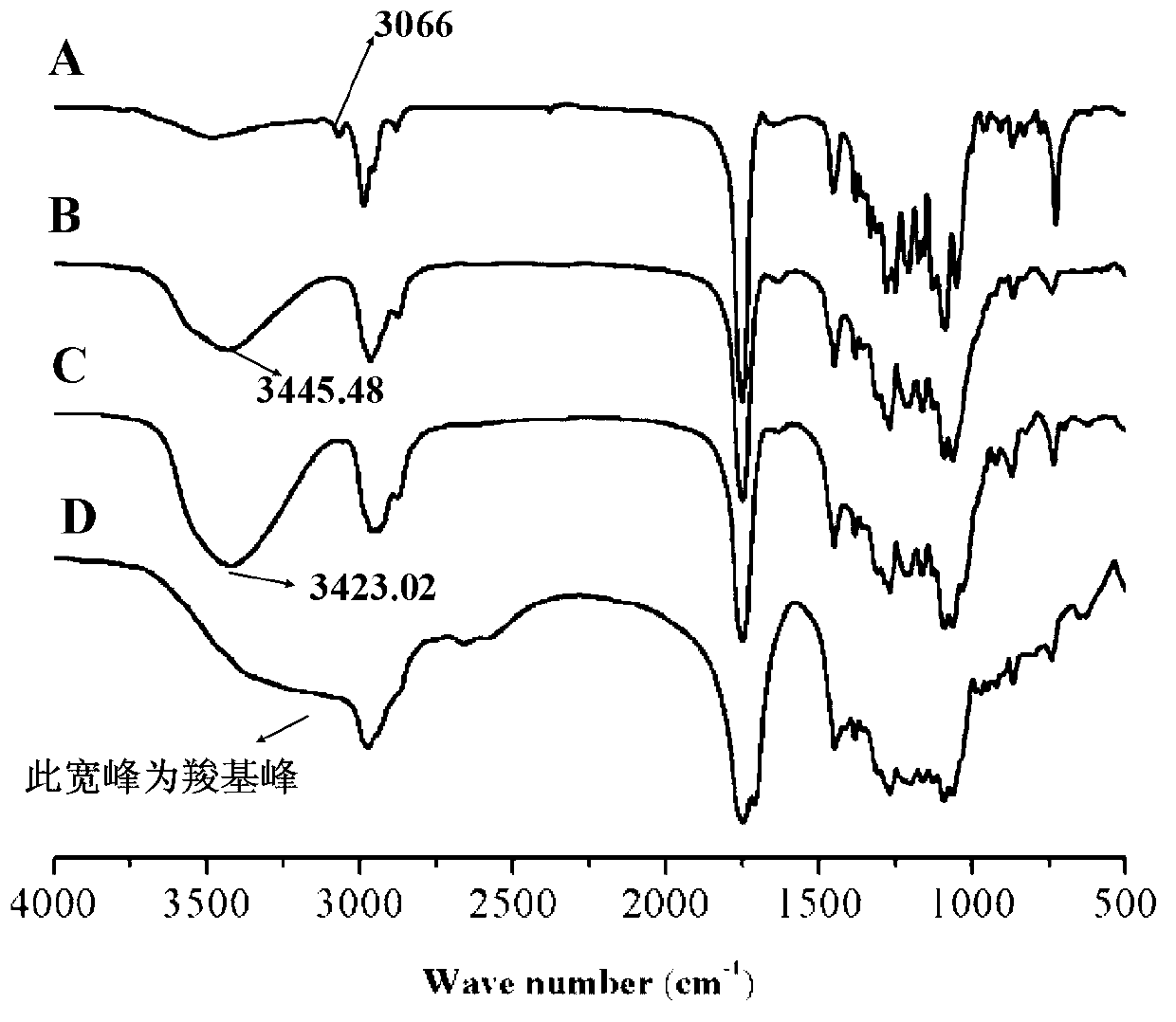
[0041] Under the protection of nitrogen, 0.100 g (double bond: 0.00048 mol) of side norbornene functionalized polylactide (NMR spectrum as shown in figure 1 ,IR image 3 Shown in A) was completely dissolved in 8 mL of tetrahydrofuran (THF), then added 0.187 g (0.0024 mol) of mercaptoethanol, and then dissolved 1-hydroxycyclohexyl phenyl ketone (photoinitiator 184) in 2 mL of THF Add it, under the irradiation of ultraviolet light with a wavelength of about 365nm, and react at room temperature for 1 hour. After the reaction, spin off THF, dissolve in dichloromethane, and precipitate in ether to obtain polylactide containing side monohydroxyl functional groups. Its structural characterization is shown in the NMR spectrum ( figure 2 ), infrared image ( image 3 B), molecular weight distribution see ( Figure 4 ), indicating that the polymer has been successfully synthesized.
Embodiment 2
[0043] Preparation of polylactide containing pendant dihydroxy functional groups:
[0044] Under the protection of nitrogen, 0.100 g (double bond: 0.00048 mol) of norbornene-functionalized polylactide was completely dissolved in 8 mL of THF, and then 0.259 g (0.0024 mol) of 3-mercapto-1 was added, 2-propanediol, and then add 1-hydroxycyclohexyl phenyl ketone dissolved in 2mL THF to it, under the irradiation of ultraviolet light with a wavelength of about 365nm, react at room temperature for 1h, after the reaction, spin off THF, and then Dissolve the crude product with a small amount of DMF, place it in a dialysis bag (3500K) and dialyze in secondary water for 48 hours to obtain polylactide containing pendant dihydroxy functional groups. Its structural characterization is shown in the NMR spectrum ( Figure 5 ), infrared image ( image 3 C), molecular weight distribution (see Figure 6 ), indicating that the polymer has been successfully synthesized.
Embodiment 3
[0046] Preparation of polylactide containing pendant monocarboxyl functional groups:
[0047] Under the protection of nitrogen, 0.100 g (double bond: 0.00048 mol) of side norbornene functionalized polylactide was completely dissolved in 8 mL of THF, then 0.254 g (0.0024 mol) of mercaptoethanol was added, and then the 2mL of THF-dissolved 1-hydroxycyclohexyl phenyl ketone was added to it, under the irradiation of ultraviolet light with a wavelength of about 365nm, the reaction was carried out at room temperature for 1h. A polylactide containing pendant monocarboxyl functional groups is obtained. Its structural characterization is shown in the NMR spectrum ( Figure 7 ), infrared image ( image 3 D), molecular weight distribution (see Figure 8 a), indicating that the polymer has been successfully synthesized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com