Method for preparing cyclohexene by benzene selective hydrogenation
A technology for selective hydrogenation and cyclohexene, applied in the fields of hydrogenation to hydrocarbons, organic chemistry, etc., can solve the problem of low selectivity, and achieve the effects of improving selectivity, increasing the conversion rate of benzene, high activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] According to the above-mentioned method for preparing cyclohexene of the present invention, the conversion rate of benzene and the selectivity of cyclohexene can be realized, but in order to better realize the purpose of the present invention, preferably, the contact between the benzene and hydrogen This is done in the presence of water and / or alcohol which favors the formation of cyclohexene. The type and addition method of the alcohol can be added at one time or in batches. The present invention has no special requirements. Preferably, the alcohol is selected from hexanediol, benzyl alcohol, α-naphthyl alcohol and 1,4-butanol One or more in diol, more preferably, described organic alcohol is the mixture of hexanediol and benzyl alcohol, to further improve the selectivity of cyclohexene, the volume ratio of hexanediol and benzyl alcohol can be in In order to better realize the purpose of the present invention, the volume ratio of hexanediol to benzyl alcohol is 0.1-1.5...
preparation Embodiment 1-6
[0047] This preparation example is used to illustrate the preparation of the hydrogenation catalyst provided by the present invention.
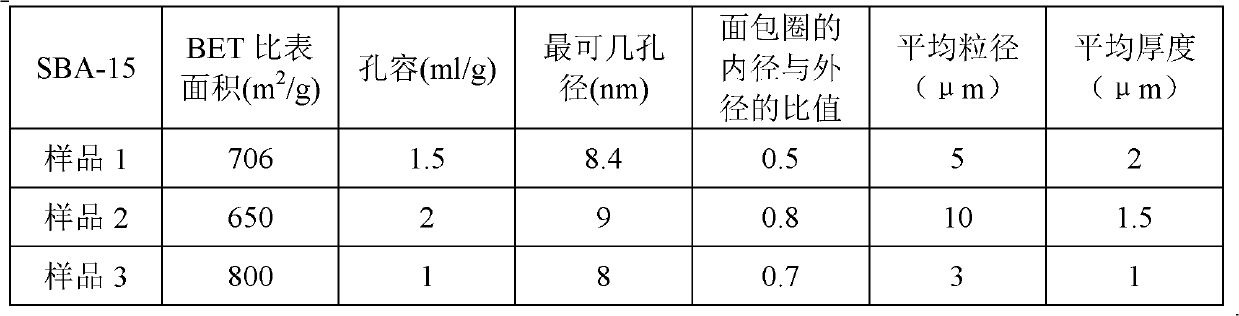
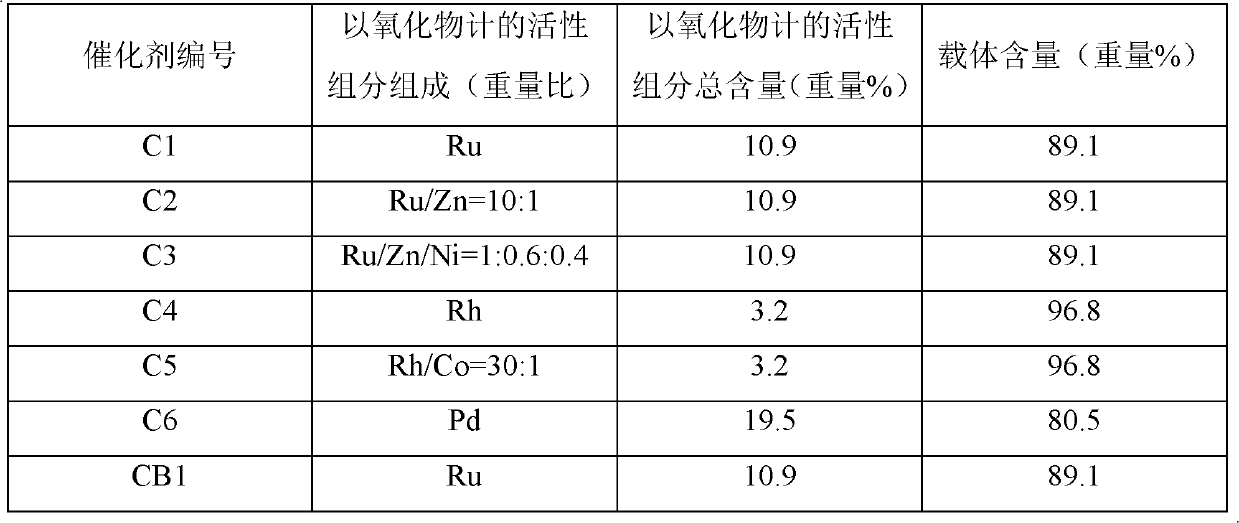
[0048] All donut-shaped SBA-15 prepared according to the aforementioned method of preparing donut-shaped SBA-15 carrier of the present invention was calcined at 400° C. for 10 hours (heat activation) under nitrogen protection to remove hydroxyl groups and residual moisture, and obtain heat-activated donut-shaped SBA-15, and hydrogenation catalysts C1-C6 were prepared according to the incipient wetness impregnation method. Adopting the incipient wetness impregnation method to prepare the hydrogenation catalyst comprises using respectively the aqueous solution of ruthenium chloride; the aqueous solution of ruthenium chloride and zinc chloride; the aqueous solution of ruthenium chloride, zinc chloride and nickel nitrate; the aqueous solution of rhodium chloride; And aqueous solution of cobalt sulfate; aqueous solution of palladium chloride for i...
Embodiment 1-6
[0057]This example is used to illustrate the use of the hydrogenation catalyst of the present invention for the selective hydrogenation of benzene to prepare cyclohexene.
[0058] The catalysts prepared in Preparation Examples 1-6 were respectively reduced in hydrogen flow at 200° C. for 3 hours to activate the catalysts. The obtained catalyst was analyzed by EPMA (X-ray microanalyzer), and it was confirmed that the active components Ru, Rh, and Pd were uniformly dispersed on the carrier. For EMPA analysis, JXA-8600M (Nippon Denshi K.K.) was used as the measuring device, the accelerating voltage of the electron gun was set to 20KV, and the probing current was 2.0×10 -8 a.
[0059] Add 120 milliliters of water, 6 grams of hydrogenation catalysts (add respectively the activated hydrogenation catalysts prepared in Examples 1-6) and 80 milliliters of benzene in the 0.5L stainless steel autoclave that has been completely purged with nitrogen in advance, and Introduce hydrogen, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com