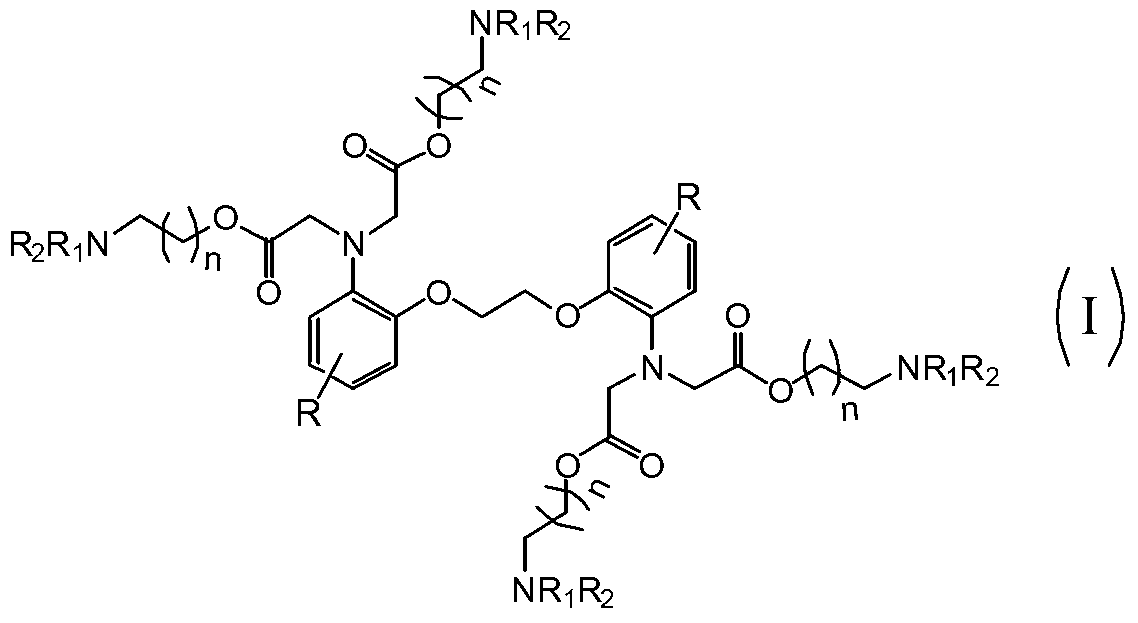
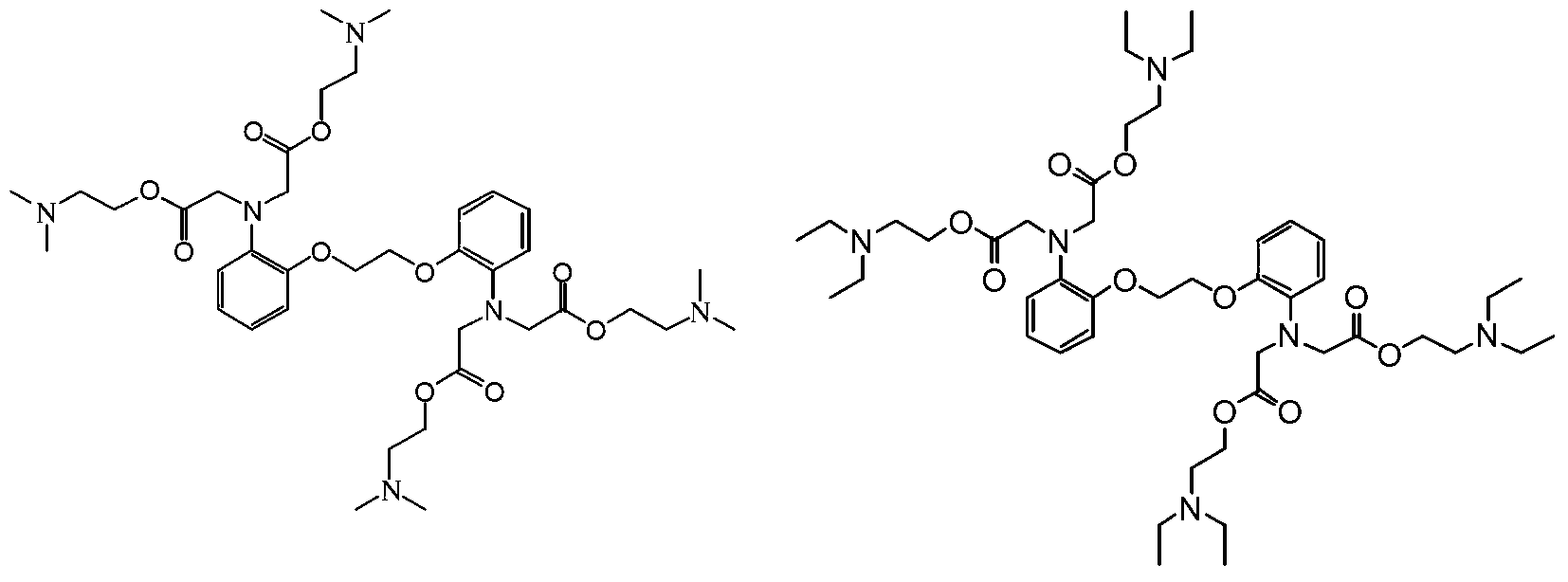
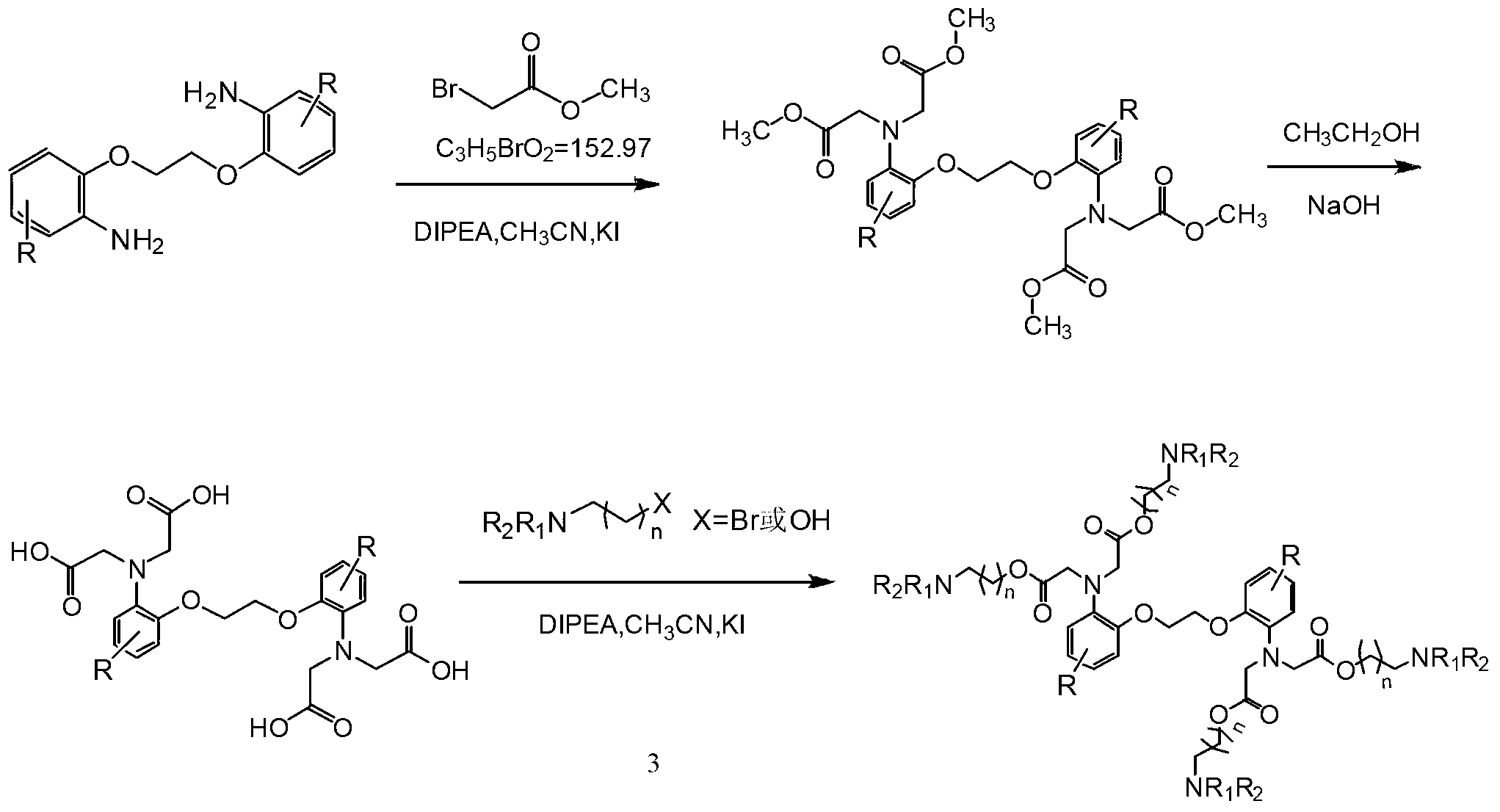
Novel BAPTA derivative, preparation method thereof and medicinal use thereof
A pharmacy, drug technology, applied in the field of improved BAPTA derivatives, which can solve problems affecting R&D and production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of BAPTA methyl ester
[0027] In a 10L dry reaction kettle, add 370g (1.5mol) of 2,2'-diaminoethylene glycol diphenyl ether and 3500ml of anhydrous acetonitrile, stir at room temperature for 10min, and 2 Under protection, 1440ml (8.32mol) of diisopropylethylamine and 70g (0.446mol) of anhydrous sodium iodide were added, and the temperature was raised slowly. When the temperature of the reaction solution reached 60°C-65°C, 768ml of methyl bromoacetate ( 8.32mol), after the dripping is completed, seal the reactor, raise the temperature to 80℃~83℃, react and stir for 27h, TLC controls the reaction end point, the developer is DMF-ethyl acetate=6:20, the reaction is completed, cooled to At room temperature, add 4000ml of toluene, stir for 10min, separate the toluene layer, continue to stir and separate the solid compound with 1000ml×4 toluene, combine the toluene layer, wash the toluene layer with 1000ml×4 deionized water, discard the water lay...
Embodiment 2
[0034] Embodiment 2: the preparation of BAPTA
[0035]In a 10L clean reaction kettle, add 532g (1.0mol) of BAPTA methyl ester and 5500ml of ethanol, heat slowly until the solids are completely dissolved, and slowly add 2667ml (5.0mol) of 15% sodium hydroxide solution [prepared by 15% sodium hydroxide solution Method: Weigh 400g of sodium hydroxide, dissolve in 2600ml of distilled water, stir to clarify, and cool to obtain], after the dropwise addition, reflux the mixture for 3 hours, TLC to control the reaction end point, the developer is DMF-ethyl acetate=1:2, After the reaction is over, cool slightly, add 15g of activated carbon, stir and reflux for 20min, remove insoluble matter by filtration, concentrate under reduced pressure to leave one-tenth of the solvent, cool to room temperature, add 1L of distilled water, cool to 5°C-10°C, use 0.1N Adjust the pH ester to 2-2.5 with hydrochloric acid, keep stirring for 20 minutes, filter, wash the solid with distilled water until th...
Embodiment 3
[0037] Example 3: Preparation of 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetra(dimethylaminoethanol)tetraacetate
[0038] In a 5000ml dry and clean four-neck flask, add BAPTA95g (0.2mol), anhydrous acetonitrile 1100ml, stir at room temperature for 10min, and 2 Under protection, add 208ml (1.2mol) of diisopropylethylamine and 12g (0.08mol) of anhydrous sodium iodide, and slowly raise the temperature. When the temperature of the reaction solution reaches 60℃~65℃, slowly drop in dimethylaminoethyl Bromine 216g (1.2mol) [Dimethylaminoethyl bromide preparation method: Take 500g of commercially available dimethylaminoethyl bromide hydrobromide, dissolve it in 1500ml distilled water, adjust the pH to 9 with saturated sodium bicarbonate solution , extracted with 3000ml of toluene, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness], after the dropwise addition, seal the reactor, raise the temperature to 80°C to 83°C, stir the reaction for 30h, and control the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com