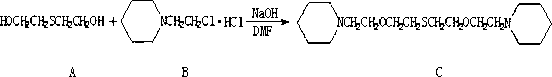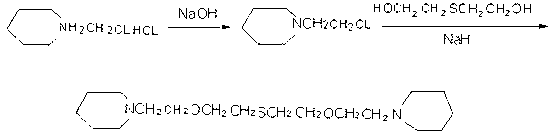Preparation method of 1,11-di(1-bispiperdine)-3,9-dioxo-6-thia-hendecane
A dioxo, dipiperidine technology, applied in the field of fine chemicals, can solve the problems of easily polluted environment, low yield, strong volatility, etc., and achieves the effects of simple post-processing process, simple process steps, and avoiding environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 3.2g (0.08mol) of NaOH and 10ml of DMF into a reaction flask equipped with a stirrer and a thermometer, add 1.22g (0.01mol) of thiodiglycol under stirring, and then add N in batches within 0.5 hours - Chloroethylpiperidine hydrochloride 4.6g (0.025mol), react at 70°C for 5 hours after addition;
[0028] (2) After the above reaction is completed, cool the reaction solution to room temperature, add 15ml of water to stir, extract with 15ml of ethyl acetate, wash with water, and wash with anhydrous MgSO 4 Concentrate after drying, and distill under reduced pressure at 195°C / 0.098Mpa to obtain 1.92g of light yellow liquid 1,11-bis(1-bispiperidine)-3,9-dioxo-6-thia-undecane , yield 56.8%, gas chromatography purity 97.6%.
Embodiment 2
[0030] (1) Add 4.0g (0.1mol) of NaOH and 15ml of DMF into a reaction flask equipped with a stirrer and a thermometer, add 1.22g (0.01mol) of thiodiethylene glycol under stirring, and then add N in batches within 0.8 hours - Chloroethylpiperidine hydrochloride 5.52g (0.03mol), react at 60°C for 6 hours after addition;
[0031] (2) After the above reaction is completed, cool the reaction solution to room temperature, add 305ml of water and stir, extract with 25ml of ethyl acetate, wash with water, and wash with anhydrous MgSO 4 Concentrate after drying, and distill under reduced pressure at 190℃ / 0.01Mpa to obtain 1.92g of light yellow liquid 1,11-bis(1-bispiperidine)-3,9-dioxo-6-thia-undecane , yield 57.2%, gas chromatography purity 98%.
Embodiment 3
[0033] (1) Add 4.0g (0.1mol) of NaOH and 20ml of DMF into a reaction flask equipped with a stirrer and a thermometer, add 1.22g (0.01mol) of thiodiethylene glycol under stirring, and then add N in batches within 1.0 hours - Chloroethylpiperidine hydrochloride 5.52g (0.03mol), react at 70°C for 6 hours after addition;
[0034] (2) After the above reaction is completed, cool the reaction solution to room temperature, add 20ml of water and stir, extract with 30ml of ethyl acetate, wash with water, and wash with anhydrous MgSO 4 Concentrate after drying, and distill under reduced pressure at 200℃ / 0.099Mpa to obtain 1.92g of light yellow liquid 1,11-bis(1-bispiperidine)-3,9-dioxo-6-thia-undecane , yield 57.6%, gas chromatography purity 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com