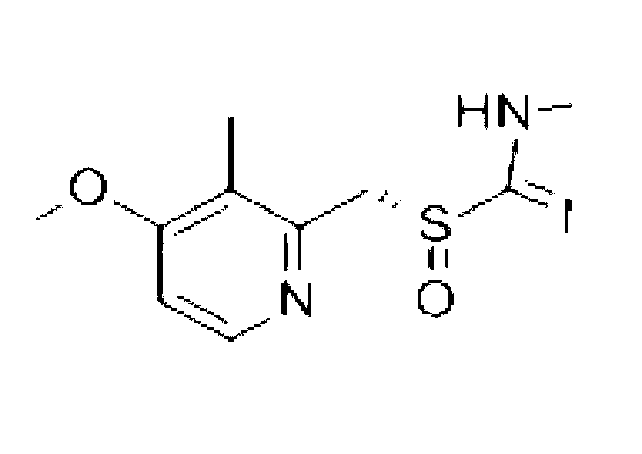
Lyophilized preparation of D-sodium lansoprazole, and preparation method thereof
A technology of dexlansoprazole sodium and freeze-dried preparations, applied in the field of pharmaceutical preparations, can solve problems such as carcinogenicity and toxic side effects, and achieve the effects of small toxic and side effects, high bioavailability, and stable products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
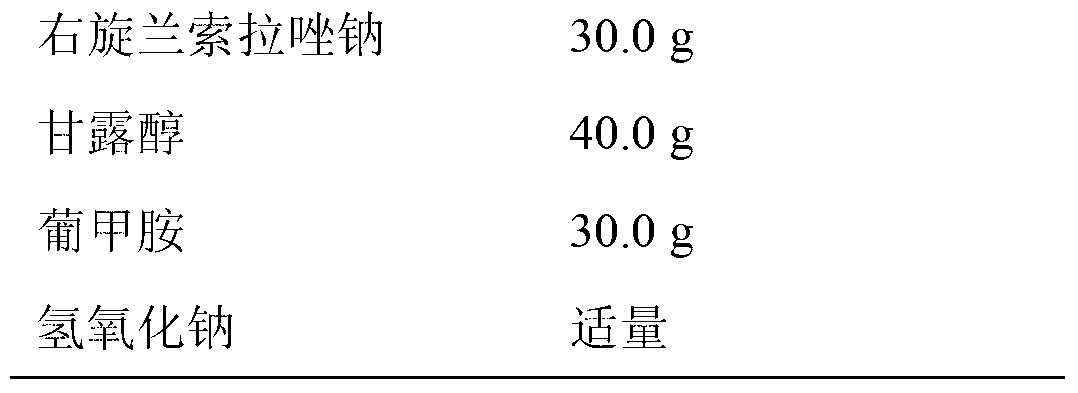
[0025] Prescription:
[0026]
[0027]
[0028] Preparation:
[0029] (1) Take 80% of the prescribed amount of water for injection, and dissolve the prescribed amount of mannitol, meglumine, and sodium biphosphite;
[0030] (2) Adjust the pH value of the solution to 10.5-12.0 with 1M sodium hydroxide solution;
[0031] (3) Ice bath the above solution to ensure that the temperature of the solution is within the range of 10°C-20°C;
[0032] (4) Add the prescribed amount of dexlansoprazole sodium and stir to dissolve;
[0033] (5) Dilute to the total volume with water for injection;
[0034] (6) Add 0.05% activated carbon, stir at room temperature for 20 minutes, filter and decarburize;
[0035] (7) Intermediate detection (pH value, content);
[0036] (8) Filling, freeze-drying and filling with nitrogen;
[0037] (9) PACKAGING.
[0038] Quality Evaluation:
[0039]
Embodiment 2
[0041] Prescription:
[0042]
[0043] Preparation:
[0044] (1) Take 80% of the prescribed amount of water for injection, and dissolve the prescribed amount of mannitol, meglumine, and sodium biphosphite;
[0045] (2) Adjust the pH value of the solution to 10.5-12.0 with 1M sodium hydroxide solution;
[0046] (3) Ice bath the above solution to ensure that the temperature of the solution is within the range of 10°C-20°C;
[0047] (4) Add the prescribed amount of dexlansoprazole sodium and stir to dissolve;
[0048] (5) Dilute to the total volume with water for injection;
[0049] (6) Add 0.05% activated carbon, stir at room temperature for 20 minutes, filter and decarburize;
[0050] (7) Intermediate detection (pH value, content);
[0051] (8) Filling, freeze-drying and filling with nitrogen;
[0052] (9) PACKAGING.
[0053] Quality Evaluation:
[0054] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com