Phosphorescent host material and preparation method thereof, and organic electroluminescent device
A technology of phosphorescent host and object materials, applied in luminescent materials, electric solid-state devices, semiconductor devices, etc., can solve the problems of low thermal stability and unsatisfactory carrier transport performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
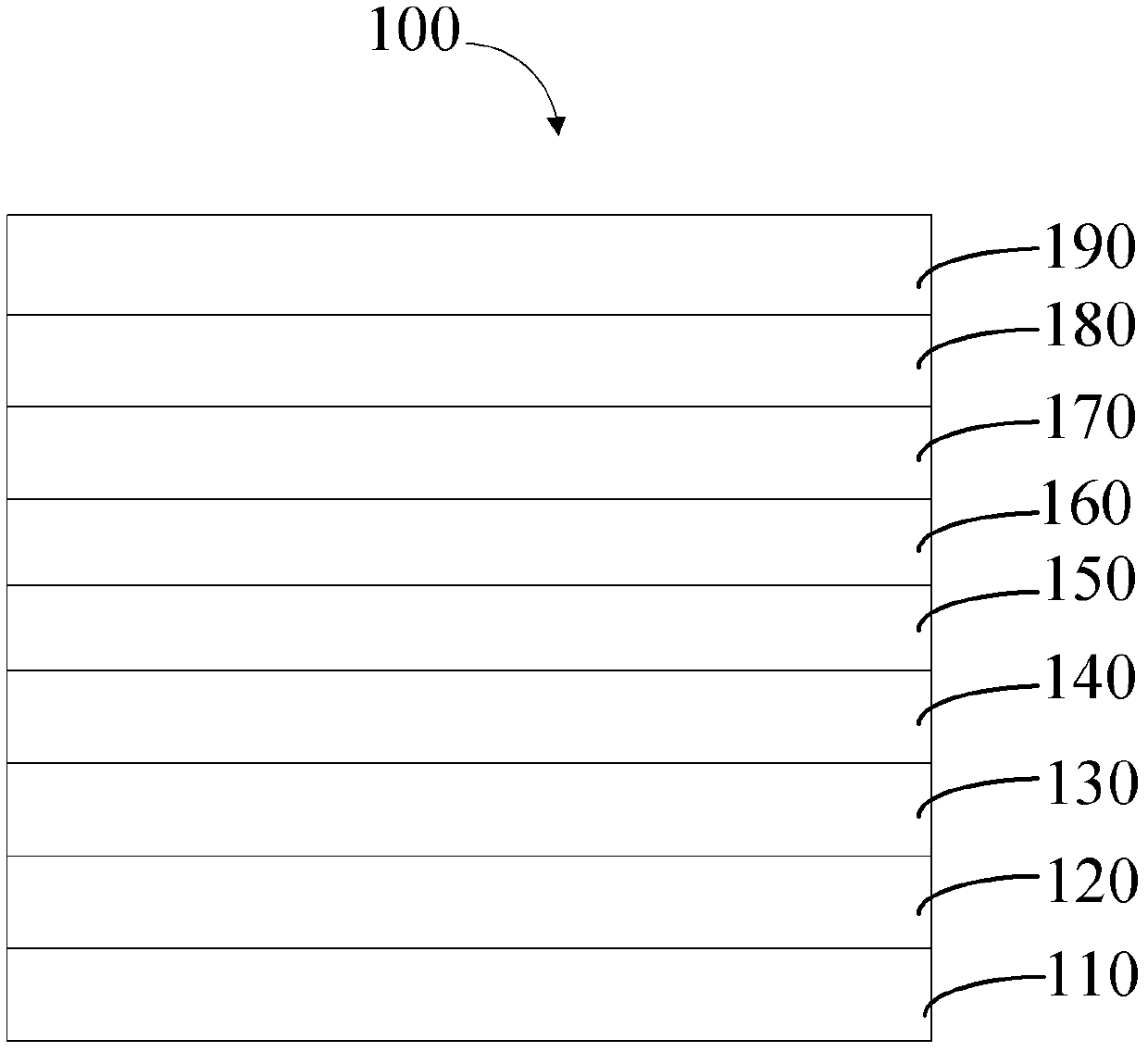
[0038] see figure 1 , a method for preparing a phosphorescent host material in an embodiment, comprising the following steps:
[0039] Step S110: In an inert gas atmosphere, add m-2,6-diformyl chloride pyridine to bromobenzene at a molar ratio of 10:1 to 15:1, stir and heat to 80°C to 100°C in the presence of the first catalyst Afterwards, the stirring reaction was continued for 2 to 4 hours to obtain 2,6-bis(4-bromobenzoyl)pyridine.
[0040] The reaction formula of step S110 is:
[0041]
[0042] After adding m-2,6-diformyl chloride pyridine to bromobenzene, then adding anhydrous aluminum trichloride (AlCl 3 ) as the first catalyst, the molar ratio of anhydrous aluminum trichloride to 2,6-diformyl chloride pyridine is 2:1-2.5:1. Stir at room temperature for 8 to 12 hours, heat to 80°C to 100°C, and continue stirring for 2 to 4 hours to generate 2,6-bis(4-bromobenzoyl)pyridine (abbreviated as 1a).
[0043] Step S110 is performed in an inert gas atmosphere such as nitrog...
Embodiment 1
[0087] Phosphorescent host material 1 with the structure shown below:
[0088]
[0089] The preparation route is as follows:
[0090]
[0091] Synthesis of 2,6-bis(4-bromobenzoyl)pyridine (1a)
[0092] Into a 250mL three-neck flask with stirring and nitrogen gas, add 15g (74mmol) m-2,6-diformyl chloride pyridine and 100mL bromobenzene at a molar ratio of 1:13, and then add 20.7g (155mmol) anhydrous Tris aluminum chloride. The molar ratio of anhydrous aluminum trichloride to m-2,6-diformyl chloride pyridine is 2.1:1. Stir at room temperature for 9 hours, then heat to 90°C and continue stirring for 2 hours. After cooling to room temperature, pour into ice-cold methanol and filter to obtain white crystals. The crude product was purified by recrystallization from toluene to obtain 2,6-bis(4-bromobenzoyl)pyridine (1a) as white crystals with a yield of 92%.
[0093] Synthesis of 2,6-bis(9-(4-bromophenyl)fluorenyl)pyridine (1b)
[0094]Add 3.49g (15mmol) 2-bromobiphenyl an...
Embodiment 2
[0098] Phosphorescent host material 2 with the structure shown below:
[0099]
[0100] Preparation of phosphorescent host material 2 with the structure shown below:
[0101]
[0102] Synthesis of 2,6-bis(4-bromobenzoyl)pyridine (1a)
[0103] In a 250mL three-necked flask with stirring and nitrogen, add 19.4g (95.5mmol) m-2,6-diformylchloride pyridine and 100mL bromobenzene in a molar ratio of 1:10, and then add 25.5g (191mmol) Aluminum trichloride water. The molar ratio of anhydrous aluminum trichloride to m-2,6-diformyl chloride pyridine is 2:1. Stir at room temperature for 9 hours, then heat to 80°C and continue stirring for 4 hours. After cooling to room temperature, pour into ice-cold methanol and filter to obtain white crystals. The crude product was purified by recrystallization from toluene to obtain 2,6-bis(4-bromobenzoyl)pyridine (1a) as white crystals with a yield of 88%.
[0104] Synthesis of 2,6-bis(9-(4-bromophenyl)fluorenyl)pyridine (1b)
[0105] Add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com