Ultra-VEGF-trap immune fusion protein, its preparation method and application
A fusion protein and protein technology, applied in the fields of peptide/protein components, chemical instruments and methods, hybrid peptides, etc., can solve the problems of reducing half-life, etc., to hinder the transfer pathway, inhibit the formation, and inhibit the development of new blood vessels and lymphatic vessels. generated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
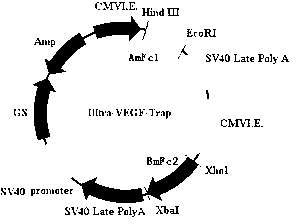
[0024] Example 1 Constructing the expression vector of the immune fusion protein Ultra-VEGF-trap
[0025] 1. Synthesis of AmFc1 and BmFc2 genes
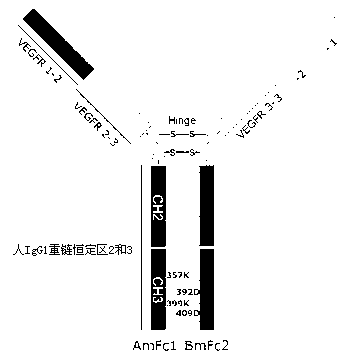
[0026] Entrusted Shanghai Jierui Bioengineering Co., Ltd. to synthesize the AmFc1 coding gene sequence, its nucleotide sequence is shown in SEQ ID.2; the amino acid sequence of AmFc1 protein is shown in SEQ ID.3, including signal peptide sequence, VEGFR1 extracellular Two regions, the third extracellular functional region of VEGFR2, and the constant region Fc of human IgG1, in which Fc has undergone two amino acid mutations, the E at position 357 is mutated to K, and the D at position 399 is mutated to K. Named AmFc1.
[0027] Also commissioned Shanghai Jierui Bioengineering Co., Ltd. to synthesize the BmFc2 coding gene sequence, the nucleotide sequence is shown in SEQ ID.4; the amino acid sequence of BmFc2 protein is shown in SEQ ID.5, including the signal peptide sequence, VEGFR3 extracellular 1 region, the 2nd functional region an...
Embodiment 2
[0031] Example 2 Expression and purification of immune fusion protein Ultra-VEGF-trap
[0032] 293F (purchased from Invitrogen, Cat No. 11625-019) cells were suspended and cultured in serum-free CD293 medium (purchased from Invitrogen, Cat No. 11913-019), centrifuged before transfection to replace fresh medium, and the cell concentration was adjusted It is 1×106 cells / ml. Taking 100ml of cells as an example, add 150μg of DNA (pT1h-ONC) and 300μg of PEI (Sigma, Cat. No: 408727) into 10ml of 293 culture medium, mix well, and let stand for 5min. After standing at room temperature for 20 minutes, the PEI / DNA suspension was added dropwise to the shake flask, mixed gently, placed in 5% CO2, 37° C. shaker culture (115 rpm) for 5 days, and the culture supernatant was collected.
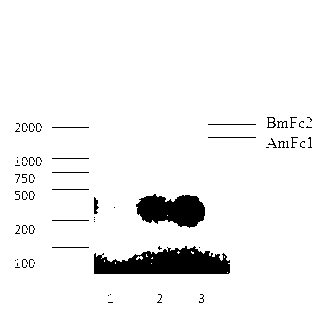
[0033] Five days after transfection, the supernatant was collected for purification. Equilibrate HiTrap MabSelectSuRe 1ml column (GE Healthcare Life Sciences product, Cat.No: 11-0034-93) 10 bed volumes with...
Embodiment 3
[0035] Example 3 Functional analysis of the immune fusion protein Ultra-VEGF-trap
[0036] VEGF165 (Beijing Hongyue Innovation Technology Co., Ltd., cat: 100-20), VEGF-B (Beijing Hongyue Innovation Technology Co., Ltd., cat: 100-20B), VEGF-C (Beijing Hongyue Innovation Technology Co., Ltd., cat: 100-20C), VEGF-D (Beijing Hongyue Innovation Technology Co., Ltd., cat: 100-20D) were diluted with 0.05mmol / L sodium carbonate, sodium bicarbonate buffer (pH 9.6) to 2μg / mL, 100μL / The wells were coated overnight at 4°C; after blocking with 1% PBS-BSA at room temperature for 1 hour, different concentrations of fusion proteins were added and incubated at room temperature for 2 hours. After washing with PBST (0.05% Tween-20) for 3 times, add 1:30000 dilution of alkaline phosphatase-labeled anti-human IgG (Zhongshan Jinqiao, cat: ZB2304), 100 μL / well, incubate at room temperature for 45 min; wash 3 times with PBST Afterwards, 100 μL / well of TMD substrate color developing solution (Kangwe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com