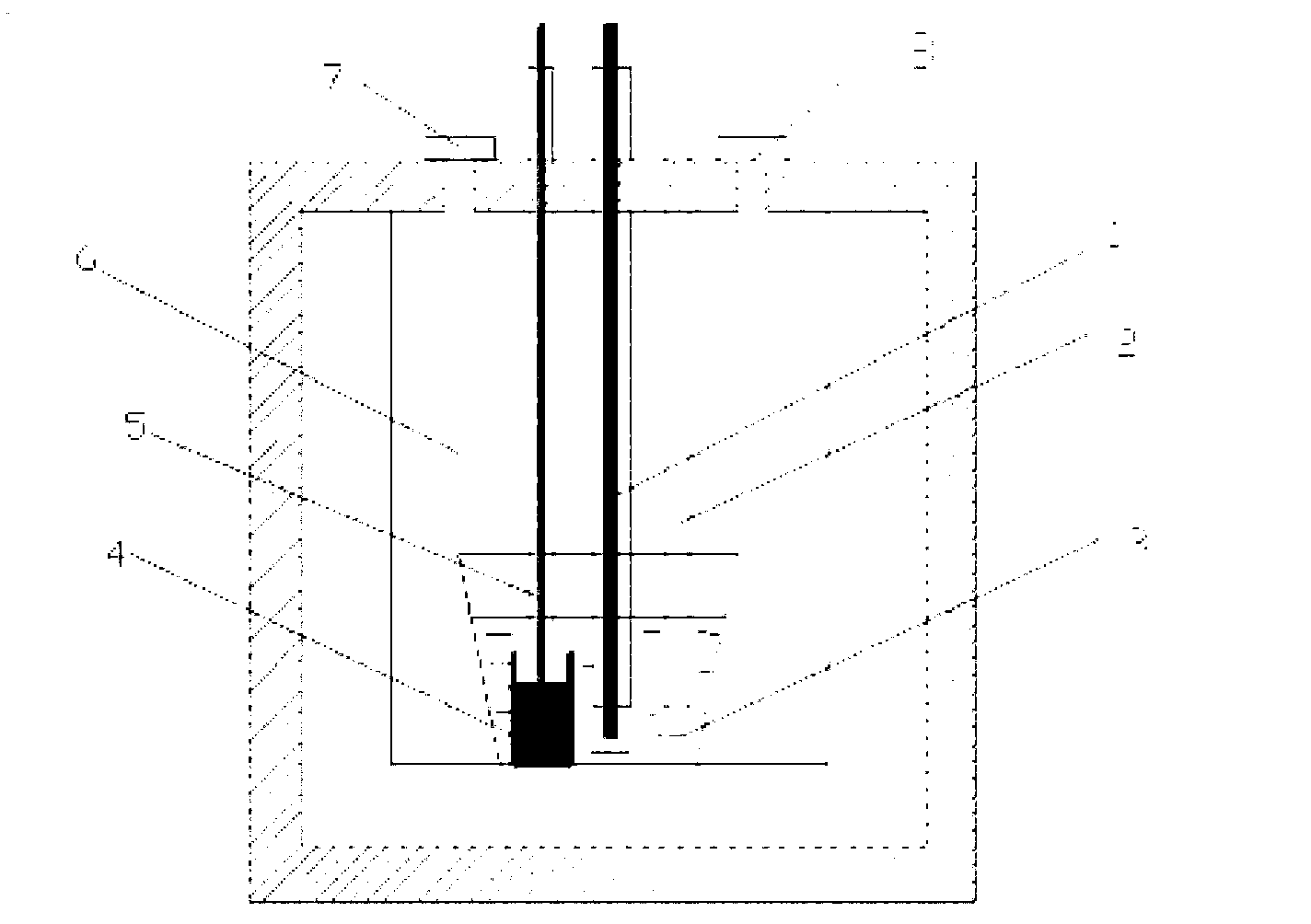
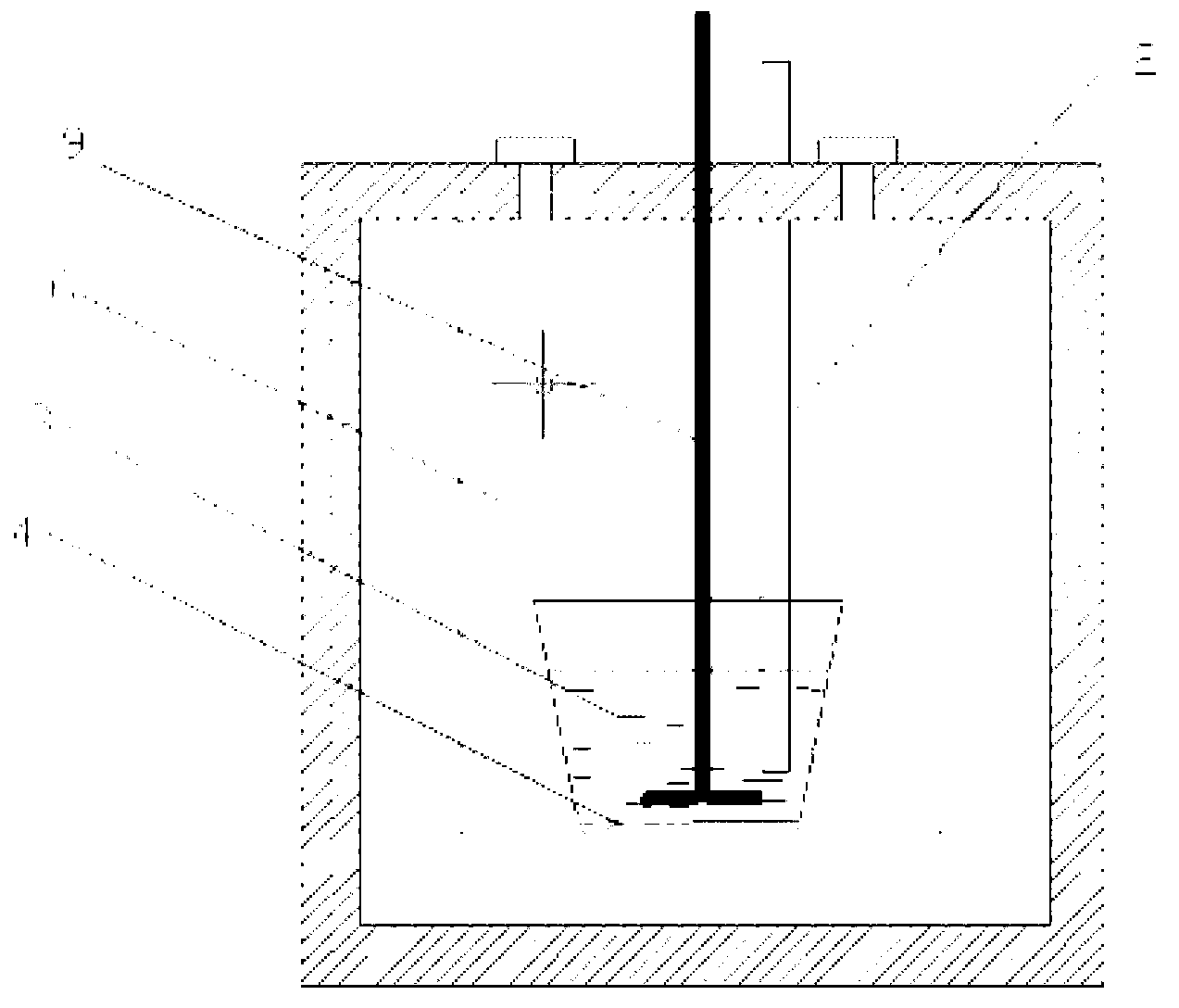
Method for extracting praseodymium and preparing aluminum-lithium-praseodymium alloy by continuous use of molten salt electrolysis and reduction extraction
A technology of molten salt electrolysis and lithium alloy, which is applied in the field of high-temperature extraction and reduction of rare earth and the production of aluminum-lithium-praseodymium alloy, which achieves wide application prospects, is conducive to the miniaturization of equipment, and the effect of small material volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1, a, preparation of reducing agent: the cathode adopts liquid aluminum, the anode adopts spectrally pure graphite rod, and the eutectic KCl-LiCl (mass fraction ratio is 51:43%) mixture is added to the electrolytic cell and heated and melted as electrolyte. , at 900 °C, galvanostatic electrolysis was performed, and the cathode current density was 1.3 A cm -2 , the tank voltage is 4.0-5.5V. After 240 minutes of electrolysis, the cathode was electrolyzed to obtain lithium, which was dissolved in liquid aluminum (5wt%) to obtain a liquid aluminum-lithium alloy; b. Extraction reaction: take out the anode and molybdenum wire, use the liquid aluminum-lithium alloy as the extractant, and the molten salt and The liquid metal ratio is 30:1, and 1wt% PrCl is added to the electrolytic cell (which doubles as an extraction cell). 3 , under the protection of argon atmosphere, stirring at 120 rpm for 4 hours; c, separation: after the reaction is complete, stop stirring, let s...
Embodiment 2
[0025] Example 2, a, preparation of reducing agent: the cathode adopts liquid aluminum, the anode adopts spectrally pure graphite rod, and the eutectic KCl-LiCl (mass fraction ratio is 52:44%) mixture is added to the electrolytic cell and heated and melted as electrolyte. , at 900 °C, galvanostatic electrolysis was performed, and the cathode current density was 1.3 A cm -2 , the tank voltage is 4.8-5.0V. After 240 minutes of electrolysis, the cathode was electrolyzed to obtain lithium, which was dissolved in liquid aluminum (3.2wt%) to obtain a liquid aluminum-lithium alloy; b. Extraction reaction: take out the anode and molybdenum wire, use the liquid aluminum-lithium alloy as the extractant, molten salt 35:1 to liquid metal, add 0.8wt% PrCl to the electrolytic cell (which doubles as an extraction cell) 3 , under the protection of argon atmosphere, stirring at 120 r / min for 5 hours; c, separation: after the reaction is complete, stop stirring, let stand for 1 hour, pour out ...
Embodiment 3
[0026] Example 3, a, preparation of reducing agent: the cathode adopts liquid aluminum, the anode adopts spectroscopically pure graphite rod, and the eutectic KCl-LiCl (mass fraction ratio is 51:43%) mixture is added into the electrolytic cell and heated and melted as electrolyte. , at 800 °C, galvanostatic electrolysis was performed, and the cathode current density was 1.3 A cm -2 , the tank voltage is 4.0-4.2V. After 240 minutes of electrolysis, the cathode was electrolyzed to obtain lithium, which was dissolved in liquid aluminum (4.8wt%) to obtain a liquid aluminum-lithium alloy; b. Extraction reaction: take out the anode and molybdenum wire, use the liquid aluminum-lithium alloy as the extractant, and melt the salt 30:1 to liquid metal, adding 1.2 wt% PrCl to the electrolytic cell (which doubles as an extraction cell) 3 , under the protection of argon atmosphere, stirring at 60 rpm for 7 hours; c, separation: after the reaction is complete, stop stirring, let stand for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com