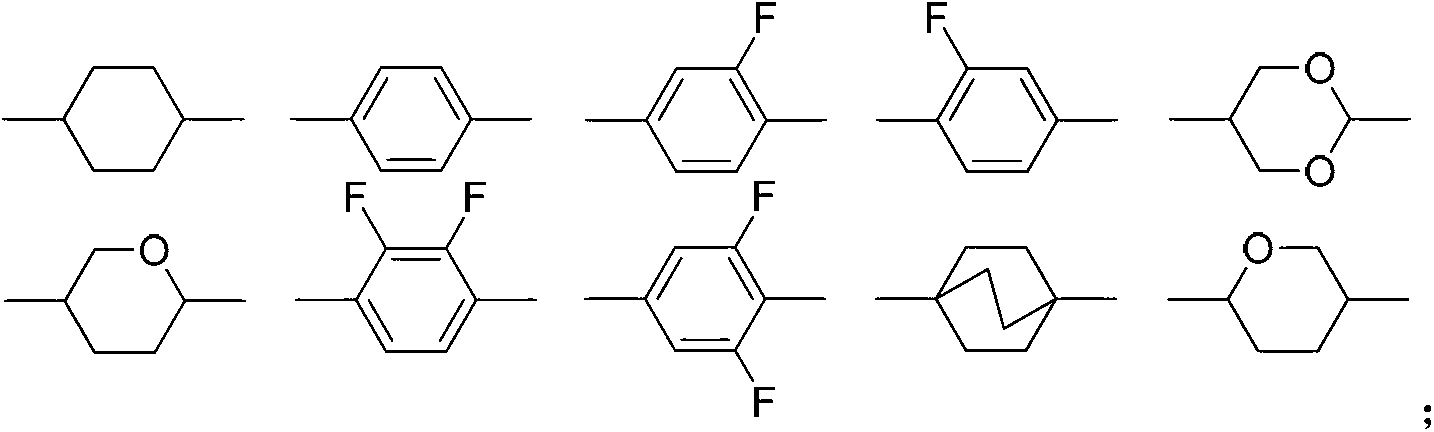
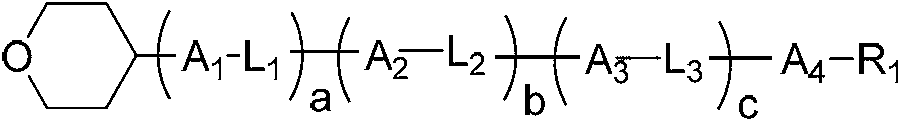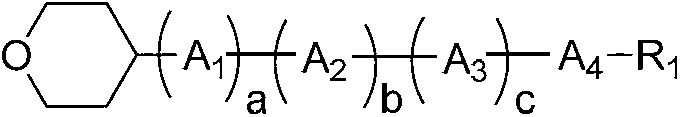Liquid crystal compound containing 4-tetrahydropyran structure and preparation method and application thereof
A compound and liquid crystal technology, applied in the field of liquid crystal compounds containing 4-tetrahydropyran structure and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1, compound shown in preparation formula I
[0115]
[0116] step 1:
[0117]
[0118] Add 56.64g (0.24mol) of 1,4-bromobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. ) n-butyllithium (reactant), dropwise and keep warm for 1 hour, under the same temperature, drop into the mixed solution of 0.216mol tetrahydropyran-4-one (reactant) and 50ml dry tetrahydrofuran (solvent), stir after dropwise After 30 minutes, the temperature was raised naturally, and 200ml of saturated ammonium chloride aqueous solution was added dropwise at about 0°C (to adjust the pH value), and the liquid was separated. The aqueous phase was extracted with 200ml of ethyl acetate (solvent), the organic phase was washed with water, and spin-dried to obtain 50g (GC: 89%) liquid, in another 1L there-necked bottle, add 50g of the product obtained above, 500ml of dry dichloromethane (solvent), under nitrogen protection, cool to -25~-20°C, add dro...
Embodiment 2
[0136] Embodiment 2, compound shown in preparation formula I
[0137]
[0138] step 1:
[0139]
[0140] Add 46.32g (0.24mol) of 3,5-difluorobromobenzene (reactant) and 400ml of dry tetrahydrofuran (solvent) into a 1L three-necked flask. 2.5N) n-butyllithium (reactant), drop and keep warm for 1 hour, under the same temperature, drop into the mixed solution of 0.216mol tetrahydropyran-4-one (reactant) and 50ml dry tetrahydrofuran (solvent), drop After stirring for 30 minutes, the temperature was raised naturally, and 200ml of saturated ammonium chloride aqueous solution was added dropwise at about 0°C (adjusting the pH value), separated, the aqueous phase was extracted with 200ml of ethyl acetate (solvent), the organic phase was washed with water, and spin-dried to obtain 50g ( GC: 89%) liquid, in another 1L three-necked bottle, add 50g of the product obtained above, 500ml of dry dichloromethane (solvent), under nitrogen protection, cool to -25~-20°C, add dropwise 63.3ml...
Embodiment 3
[0157] Embodiment 3, compound shown in preparation formula I
[0158]
[0159] step 1
[0160]
[0161] Specifically with step 1 in the above embodiment 1.
[0162] step 2
[0163]
[0164] Add 0.1mol (3-a) (reactant) and 120ml tetrahydrofuran (solvent) obtained in the step into the reaction flask, install and seal the stirring, replace the air with nitrogen, cool to -70°C, and add dropwise 0.1mol concentration of 2.5M butane Lithium-based (reactant), 20 minutes after the addition, feed dry carbon dioxide gas (reactant), to saturation, react at this temperature for 2 hours, pour this reaction solution into 20ml concentrated hydrochloric acid (adjust pH value) Hydrolyze in a beaker with 100ml of water, separate the liquids, extract the water phase once with 50ml of ethyl acetate (solvent), combine the organic phases, wash with saturated brine until neutral, dry over anhydrous sodium sulfate (desiccant), concentrate to remove the solvent, and obtain Light yellow soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com