A thrombolytic liquid preparation capable of avoiding fibrinogen degradation and a preparation method thereof
A protein source and liquid embolization technology, applied in the field of medicine, can solve problems such as bleeding, and achieve the effects of good repeatability, reduced bleeding risk rate, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
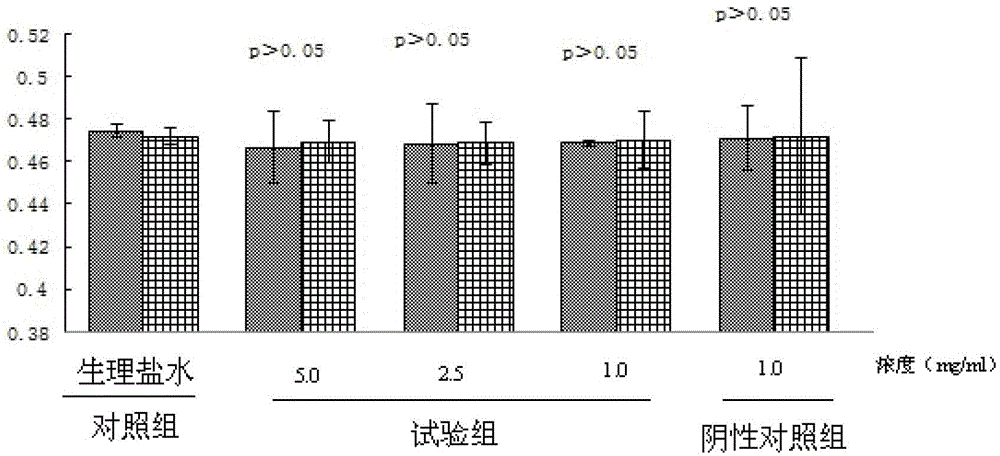
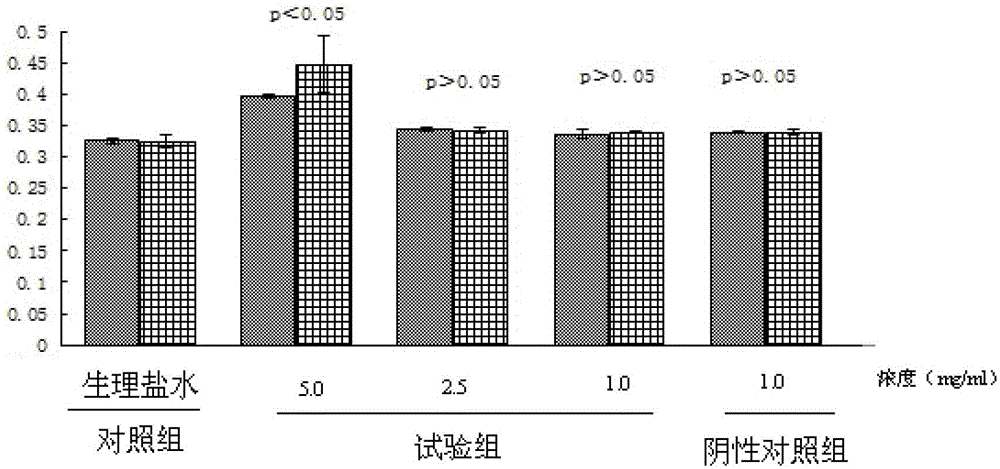
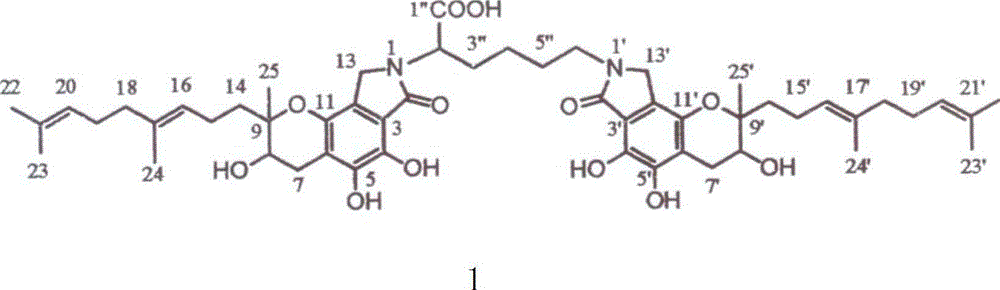
[0038] Experimental materials: 30 Wistar rats, FGFC1 50mg, sodium bicarbonate 50mg, 0.85% normal saline
[0039]The specific operation of the experiment is as follows: (1) Solution preparation: Weigh FGFC1, then weigh an equivalent amount of co-solvent sodium bicarbonate, add 10ml of normal saline to stir and dissolve, so that the concentration of FGFC1 in the final prepared solution is 5.0mg / ml; ( 2) Filtration: Filter and sterilize the prepared solution with a 0.2 μm microporous membrane, measure the content of FGFC1 in the solution again, put it into a brown bottle after passing the test, and avoid light. (3) Intravenous injection: Wipe the tail of the rat with alcohol cotton to dilate the vein for injection, and then inject through the tail vein according to the ratio of 2ml / 20g. The injection speed is slow to avoid the death of the rat due to heart failure. (4) Blood collection: sacrificed and dissected at 30 minutes and 2 hours respectively, blood was collected from the ...
Embodiment 2
[0041] Experimental materials: 30 Wistar rats, FGFC1 10mg, sodium bicarbonate 10mg, 0.85% normal saline
[0042] The specific operation of the experiment is as follows: (1) Solution preparation: weigh FGFC1, then weigh an equivalent amount of co-solvent sodium bicarbonate, add 4ml of normal saline to stir and dissolve, so that the concentration of FGFC1 in the final prepared solution is 2.5mg / ml; ( 2) Filtration: Filter and sterilize the prepared solution with a 0.2 μm microporous membrane, measure the content of FGFC1 in the solution again, put it into a brown bottle after passing the test, and avoid light. (3) Intravenous injection: Wipe the tail of the rat with alcohol cotton to dilate the vein for injection, and then inject through the tail vein according to the ratio of 2ml / 20g. The injection speed is slow to avoid the death of the rat due to heart failure. (4) Blood collection: sacrificed and dissected at 30 minutes and 2 hours respectively, blood was collected from the ...
Embodiment 3
[0044] Experimental materials: 30 Wistar rats, FGFC1 10mg, sodium bicarbonate 10mg, 0.85% normal saline
[0045] The specific operation of the experiment is as follows: (1) Solution preparation: weigh FGFC1, then weigh an equivalent amount of co-solvent sodium bicarbonate, add 10ml of normal saline to stir and dissolve, so that the concentration of FGFC1 in the final prepared solution is 1.0mg / ml; ( 2) Filtration: Filter and sterilize the prepared solution with a 0.2 μm microporous membrane, measure the content of FGFC1 in the solution again, put it into a brown bottle after passing the test, and avoid light. (3) Intravenous injection: Wipe the tail of the rat with alcohol cotton to dilate the vein for injection, and then inject through the tail vein according to the ratio of 2ml / 20g. The injection speed is slow to avoid the death of the rat due to heart failure. (4) Blood collection: sacrificed and dissected at 30 minutes and 2 hours respectively, blood was collected from the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com