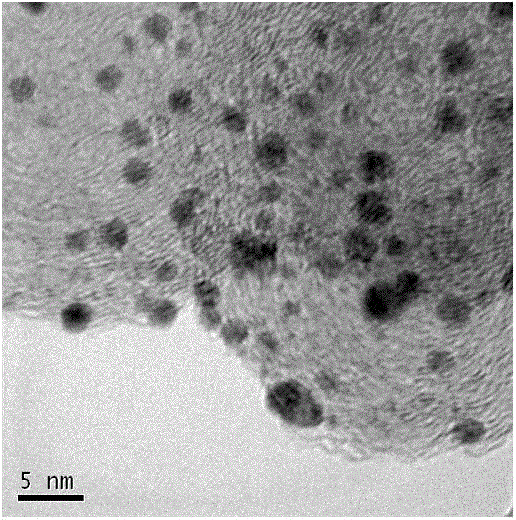
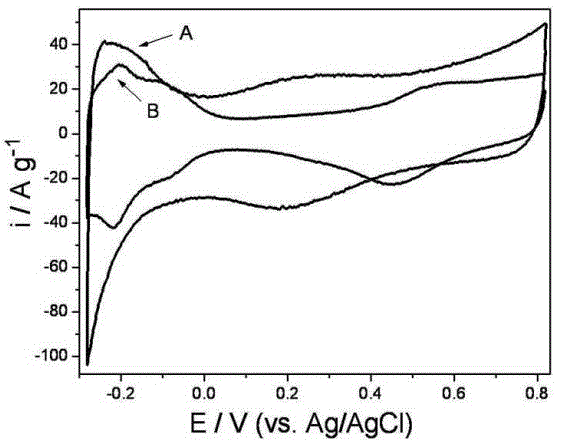
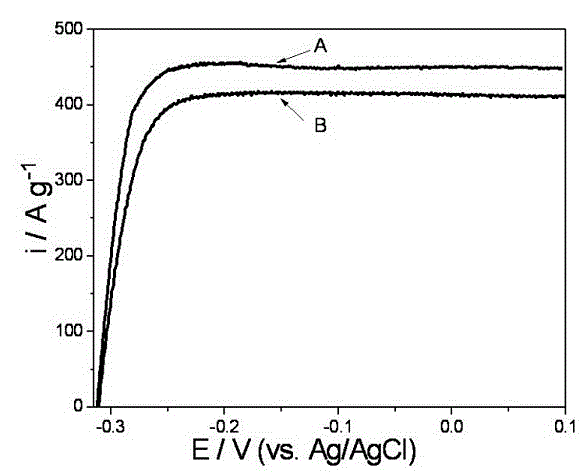
Preparation method for non-Pt non-H anode catalyst of proton exchange membrane fuel cell (PEMFC)
A proton exchange membrane and fuel cell technology, applied in battery electrodes, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., to achieve large-scale commercial production, high-efficiency catalytic hydrogen oxidation performance, and methods simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Functionalization of carbon supports
[0034] Take a 500ml round bottom flask, weigh 1 gram of commercially available Vulcan XC-72 carbon powder, add 150ml of a mixed solution of 30% hydrogen peroxide:concentrated sulfuric acid with a volume ratio of 1:4, ultrasonicate and stir for 3 hours, then use ultra-pure Dilute it with water, let it stand for 24 hours, filter out the supernatant, and get the functionalized Vulcan XC-72 carbon powder after several times of centrifugal washing, drying, and grinding.
[0035] (2) Preparation of carbon-supported iridium-nickel complexes
[0036] According to the mass ratio of functionalized Vulcan XC-72 carbon powder: chloroiridic acid: nickel chloride: sodium citrate 1: 0.45: 0.13: 1.17, respectively weigh the functionalized Vulcan XC-72 carbon powder and iridium chloride obtained in step (1) acid, nickel chloride and sodium citrate; first add the functionalized Vulcan XC-72 carbon powder into deionized water, and ultrasonically...
Embodiment 2
[0053] Step (1) is the same as step (1) in Example 1.
[0054] (2) Preparation of carbon-supported iridium-nickel complexes
[0055] According to the mass ratio of functionalized Vulcan XC-72 carbon powder: sodium chloroiridate: nickel nitrate: sodium citrate 1︰0.51︰0.11︰1, weigh the functionalized Vulcan XC-72 carbon powder and iridium chloride obtained in step (1) sodium citrate, nickel nitrate and sodium citrate; first add the functionalized Vulcan XC-72 carbon powder into deionized water, and ultrasonically disperse for 30 minutes to form a uniformly dispersed functionalized Vulcan XC-72 carbon powder with a mass concentration of 15 mg / ml Suspension; Then add sodium chloroiridate, nickel nitrate and sodium citrate successively, first ultrasonically disperse for 20 minutes, continue to stir for 15 hours, then adjust the pH value to 11.5 with mass concentration of 28% ammonia water, seal and stir for 15 hours, then Dry it in a water bath at 60°C and grind it into powder to ...
Embodiment 3
[0062] Step (1) is the same as step (1) in Example 1
[0063] (2) Preparation of carbon-supported iridium-nickel complexes
[0064] Weigh the functionalized Vulcan XC-72 carbon powder, chlorine Sodium iridite, nickel sulfate and sodium citrate; first add functionalized Vulcan XC-72 carbon powder to deionized water, and ultrasonically disperse for 60 minutes to form a uniformly dispersed functionalized Vulcan XC-72 with a mass concentration of 25 mg / ml Carbon powder suspension; then add sodium chloroiridite, nickel sulfate and sodium citrate in sequence, ultrasonically disperse for 10 minutes, continue stirring for 6 hours, then adjust the pH value to 11 with ammonia water with a mass concentration of 28%, and seal and stir for 6 hours hours, then dried in a water bath at 50°C, and ground into powder to obtain a carbon-supported iridium-nickel complex.
[0065] (3) Preparation of carbon-supported iridium-nickel alloy catalyst
[0066] The carbon-supported iridium-nickel comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com