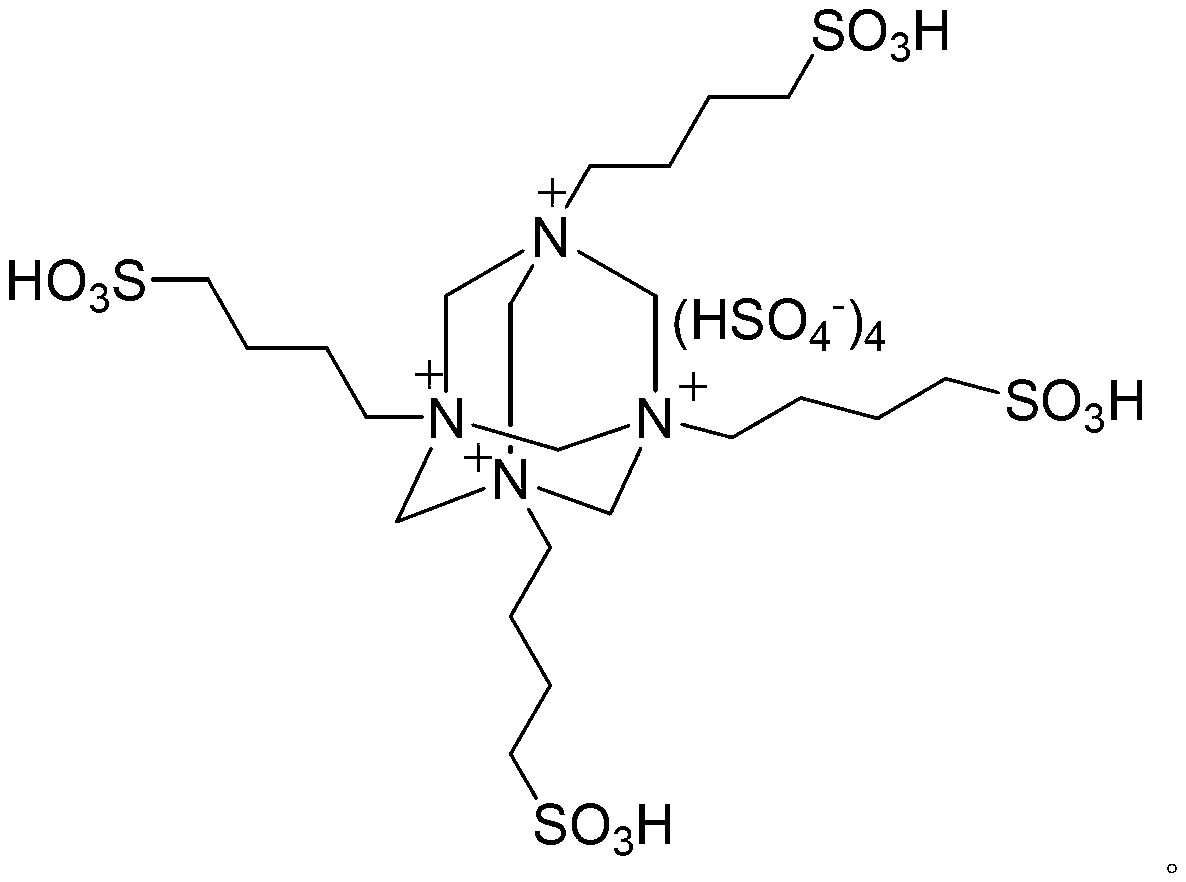
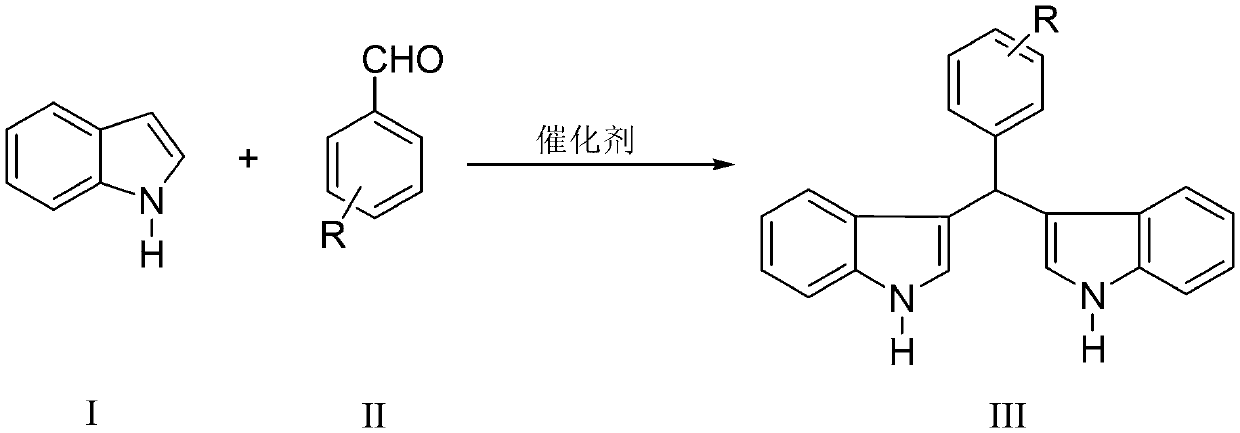
Preparation method of diindolylmethane derivative through catalysis
A technology for the catalytic preparation of diindolylmethane, which is applied in the field of catalytic preparation of diindolylmethane derivatives, can solve the problems of high cost and complex preparation of acidic ionic liquids, and achieve environmental pollution reduction, high yield, and good catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Add 2 mmol of indole, 1 mmol of benzaldehyde, 0.01 mmol of catalyst and 2 mL of water into a 25 mL single-neck flask with a stir bar. The reaction was stirred vigorously at 25°C, and thin-layer chromatography (TLC) (developing solvent: n-hexane: ethyl acetate=4:1) followed the progress of the reaction. When the reaction is applied for 6 minutes, the reaction pressure is one atmosphere. After the completion of the reaction, suction filtration, the obtained filter residue was recrystallized with 98% ethanol aqueous solution and dried to obtain pure diindophenylmethane with a yield of 93%. The filtrate (including catalyst and unreacted raw materials) is directly used in the next reaction without any treatment.
Embodiment 2
[0020] Example 2: Add 2 mmol of indole, 1 mmol of p-chlorobenzaldehyde, 0.01 mmol of catalyst, and 2 mL of water into a 25 mL single-necked flask with a stir bar. The reaction was stirred vigorously at 25°C, and thin-layer chromatography (TLC) (developing solvent: n-hexane: ethyl acetate=4:1) followed the progress of the reaction. When the reaction is applied for 8 minutes, the reaction pressure is one atmosphere. After the reaction, the filter residue was recrystallized with a 98% ethanol aqueous solution and dried to obtain pure p-chlorophenyl diindolylmethane with a yield of 94%. The filtrate (including catalyst and unreacted raw materials) is directly used in the next reaction without any treatment.
Embodiment 3
[0021] Example 3: Add 2 mmol of indole, 1 mmol of p-nitrobenzaldehyde, 0.01 mmol of catalyst and 4 mL of water into a 25 mL single-neck flask with a stir bar. The reaction was stirred vigorously at 27° C., and thin-layer chromatography (TLC) (developing solvent: n-hexane: ethyl acetate=4:1) followed the progress of the reaction. When the reaction is applied for 3 minutes, the reaction pressure is one atmosphere. After the completion of the reaction, suction filtration, the obtained filter residue was recrystallized with 98% ethanol aqueous solution and dried to obtain pure p-nitrophenyl diindolylmethane with a yield of 97%. The filtrate (including catalyst and unreacted raw materials) is directly used in the next reaction without any treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com