Ractopamine aptamer and electrochemical biosensor for detecting same
A technology of ractopamine and biosensor, applied in the field of detection, can solve the problems of high cost of ractopamine detection, long detection time, complex detection scheme, etc., to reduce detection cost and complexity of detection scheme, low detection cost, and convenient modification fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Construction of an aptamer electrochemical biosensor
[0037] (1) Polishing and activation treatment of bare gold electrode surface
[0038] Grind the bare gold electrode with 0.3 μm alumina powder for 10 minutes, then polish it with 0.05 μm alumina powder for 20 minutes, and then ultrasonically clean it twice with ultrapure water for 5 minutes each time to completely remove the non-specific adsorption on the bare gold electrode. Aluminum oxide powder on the electrode surface. Immerse the cleaned bare gold electrode in freshly prepared hot Piranha solution (concentrated sulfuric acid: 30% H 2 o 2 =7:3, V:V) were activated at room temperature for 10 minutes to obtain activated bare gold electrodes. Then ultrasonic cleaning was performed twice with ultrapure water and absolute ethanol, 5 minutes each time.
[0039] (2) Modification of aptamer electrochemical sensors
[0040] Take 2 μL of ractopamine aptamer solution with a concentration of 100 μmol / L and a...
Embodiment 2
[0043] Example 2: Electrochemical response of ractopamine aptamer electrochemical biosensor to ractopamine standard solution
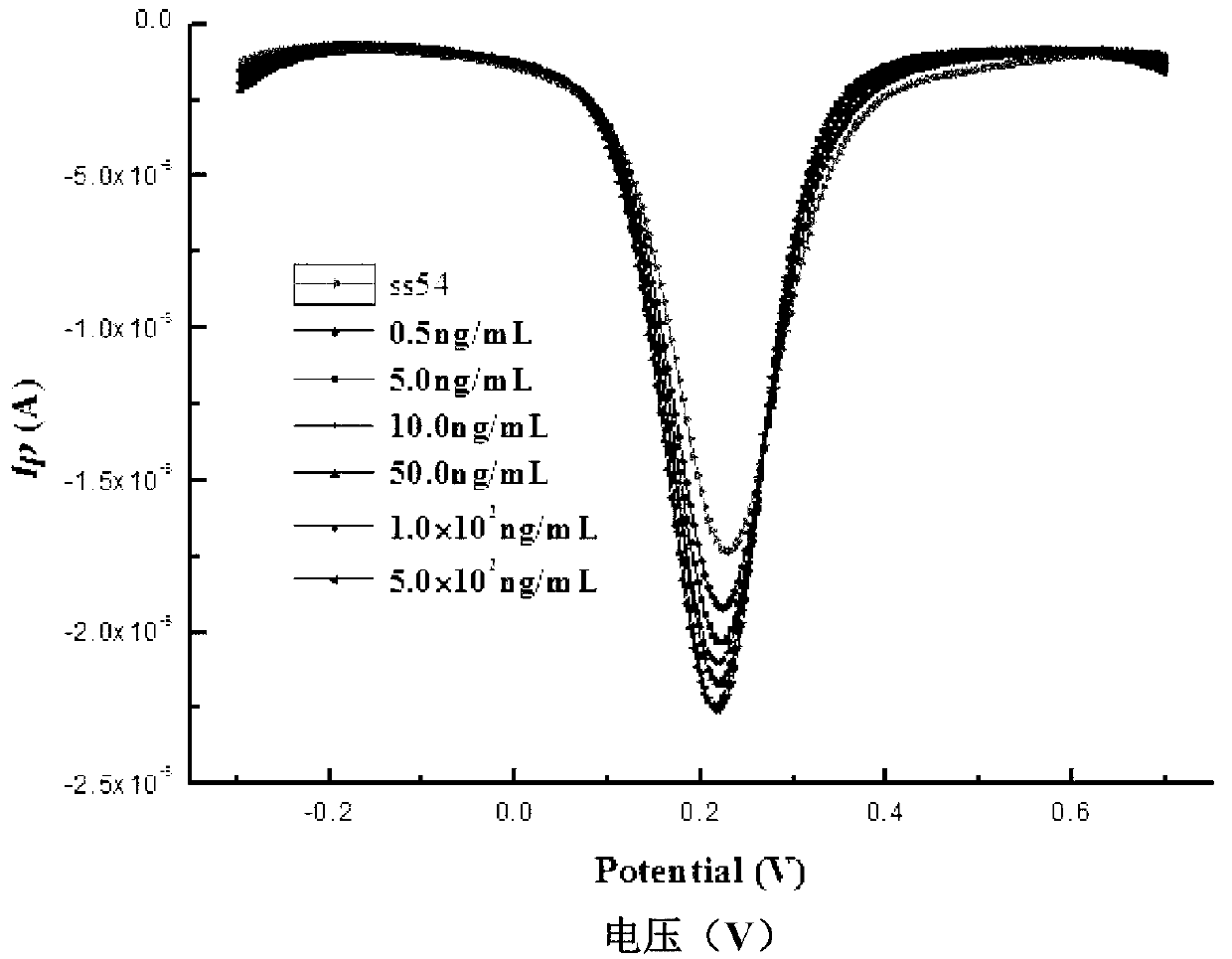
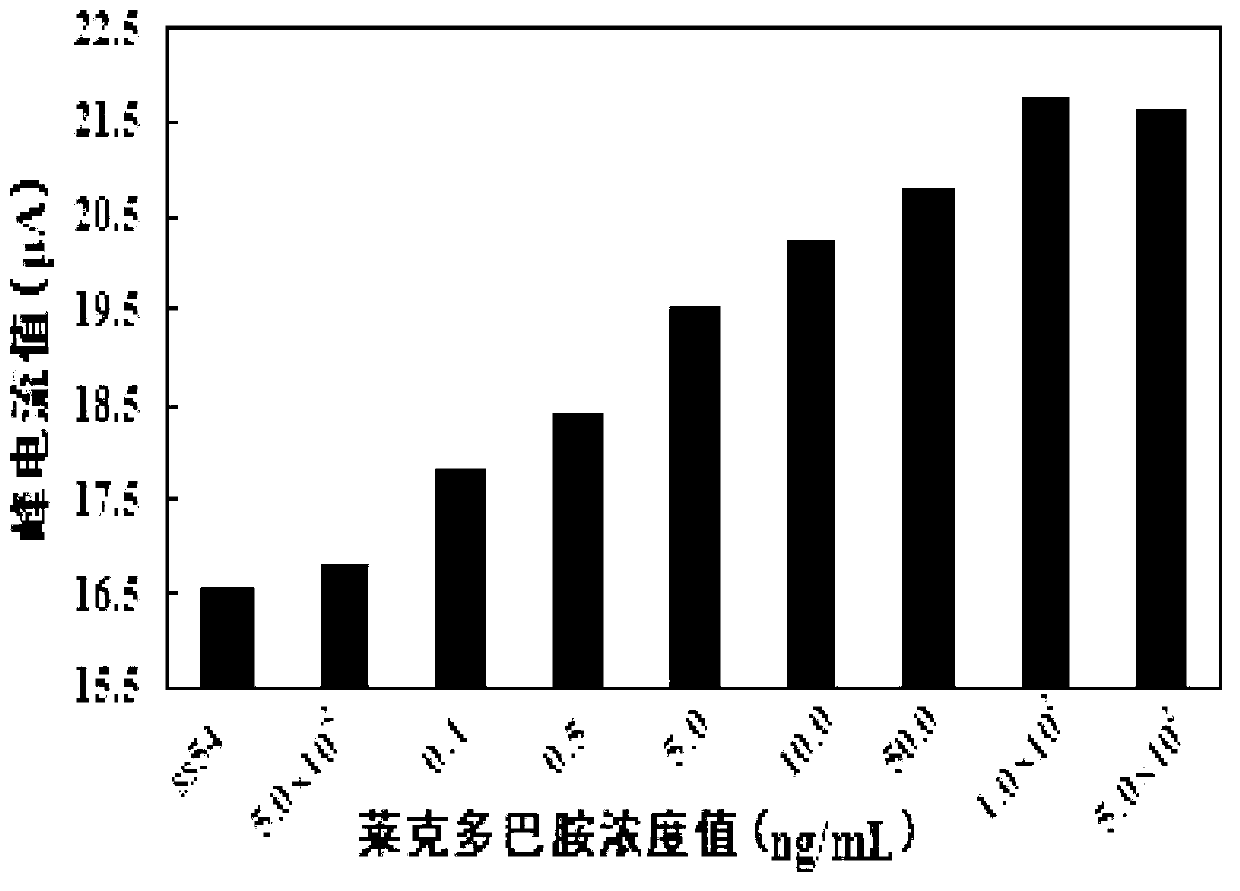
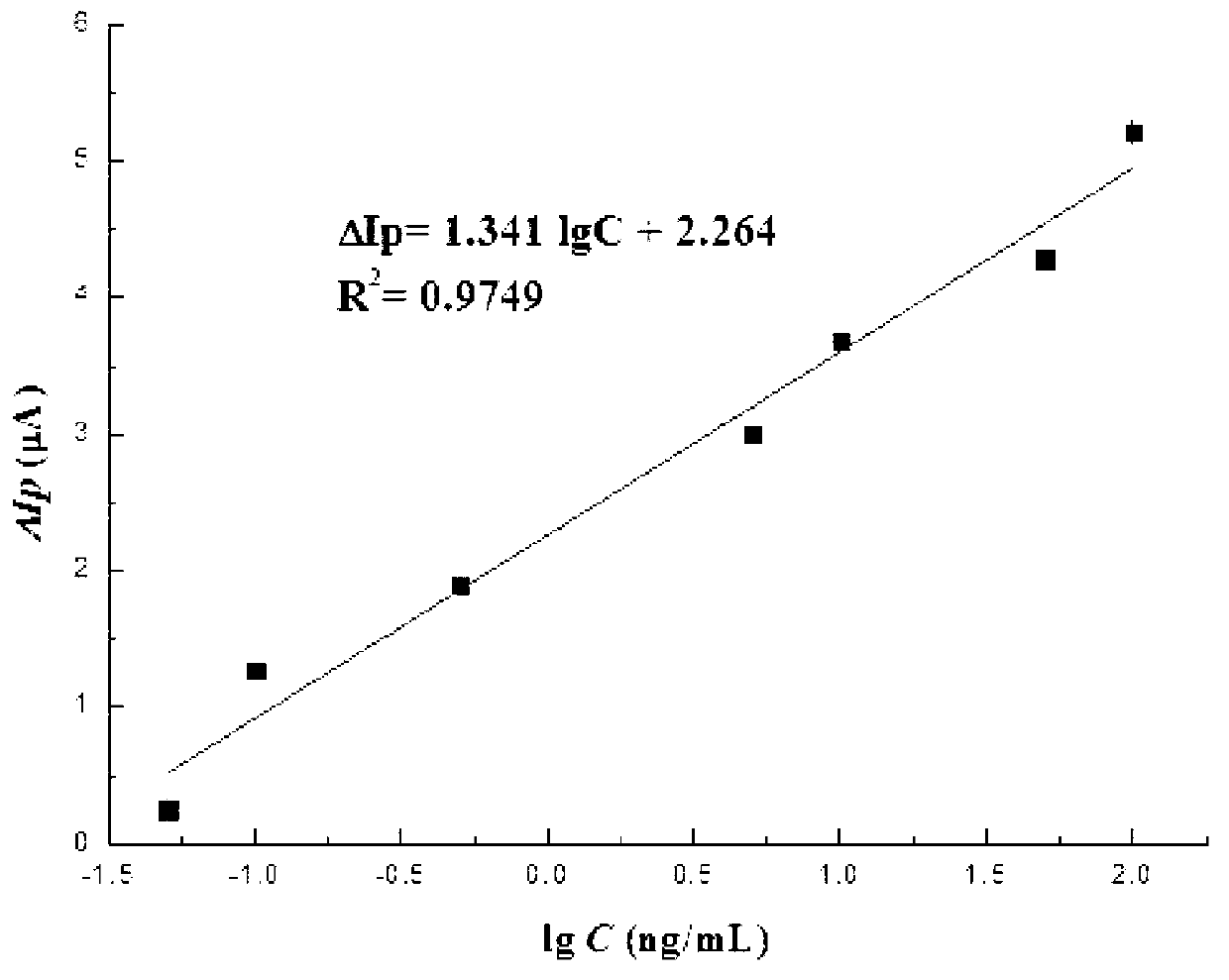
[0044] (1) The electrochemical nucleic acid aptamer electrochemical biosensor produced in Example 1 was used as the working electrode, the Ag / AgCl (saturated KCl) electrode was used as the reference electrode, and the platinum wire electrode was used as the counter electrode. The electrochemical nucleic acid aptamer electrochemical biosensor made in Example 1 was rinsed with ultrapure water to remove unbound aptamers, and then placed in a concentration of 5 mmol / L K 3 [Fe(CN) 6 ] In the electrolyte, the electrolyte contains KCl with a concentration of 0.1mol / L. Differential pulse voltammetry analysis shows that the high potential of scanning is 0.7V, the low potential is 0.3V, the scanning speed is 0.02V / s, and the amplitude is 0.05V. DPV spectrum of ligand sensor and its peak current value Ip 0 .
[0045] (2) Rinse the aptamer sensor described in s...
Embodiment 3
[0048] Example 3: Quantitative detection of compound feed containing ractopamine
[0049] Treatment of compound feed samples: Take 5.00 g of compound feed samples, add different concentrations of ractopamine, and extract and purify the ractopamine in the compound feed according to the method described in GB / T22147-2008. The obtained ractopamine dry powder was dissolved in ultrapure water, passed through a 0.45 μM filter membrane, and then electrochemically detected.
[0050] The electrochemical nucleic acid aptamer sensor made in Example 1 was rinsed with ultrapure water to remove unbound aptamers, and then placed in K with a concentration of 5 mmol / L. 3 [Fe(CN) 6 ] In the electrolyte, the electrolyte contains KCl with a concentration of 0.1mol / L. Differential pulse voltammetry analysis shows that the high potential of scanning is 0.7V, the low potential is 0.3V, the scanning speed is 0.02V / s, and the amplitude is 0.05V. DPV spectrum of ligand sensor and its peak current val...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com