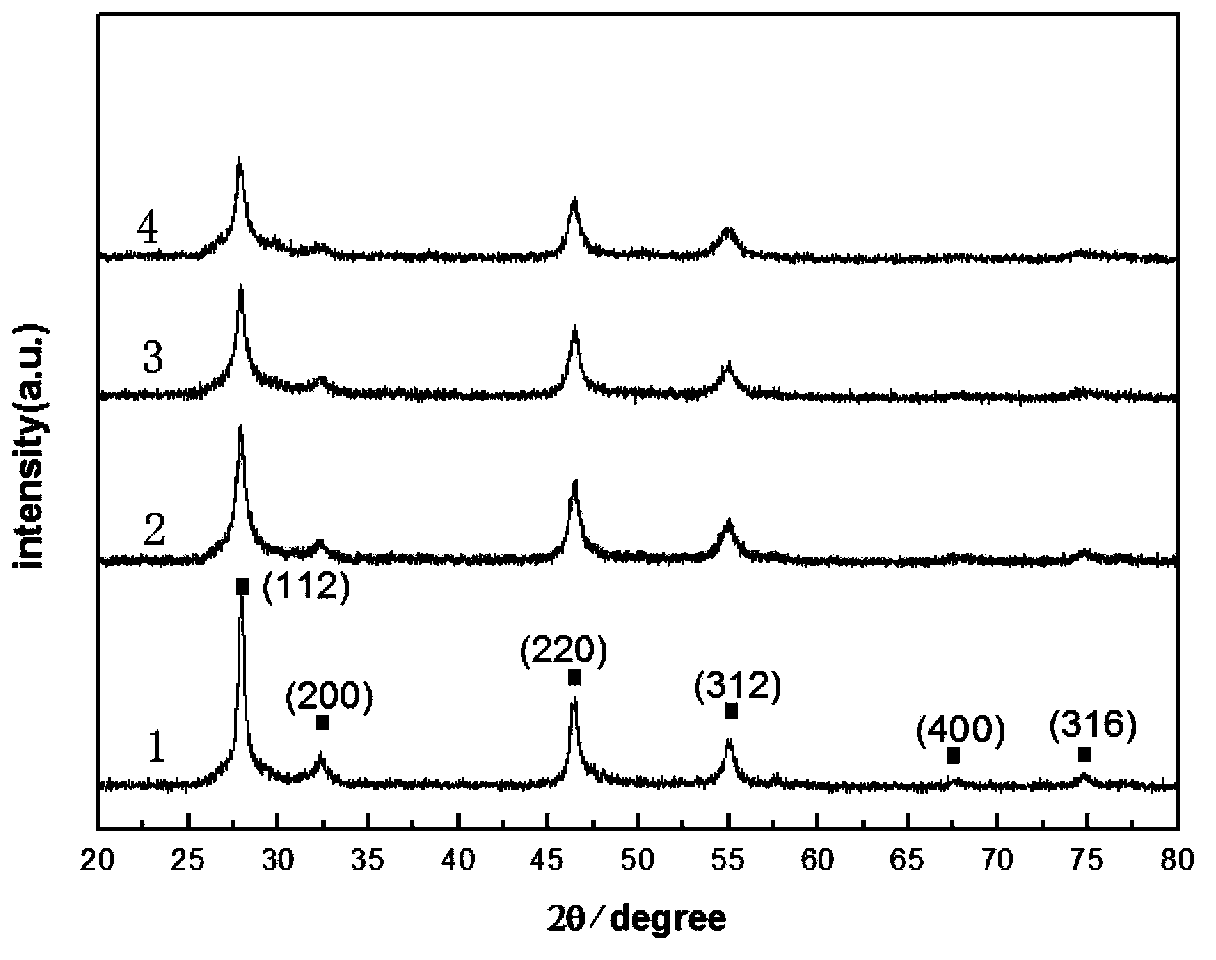
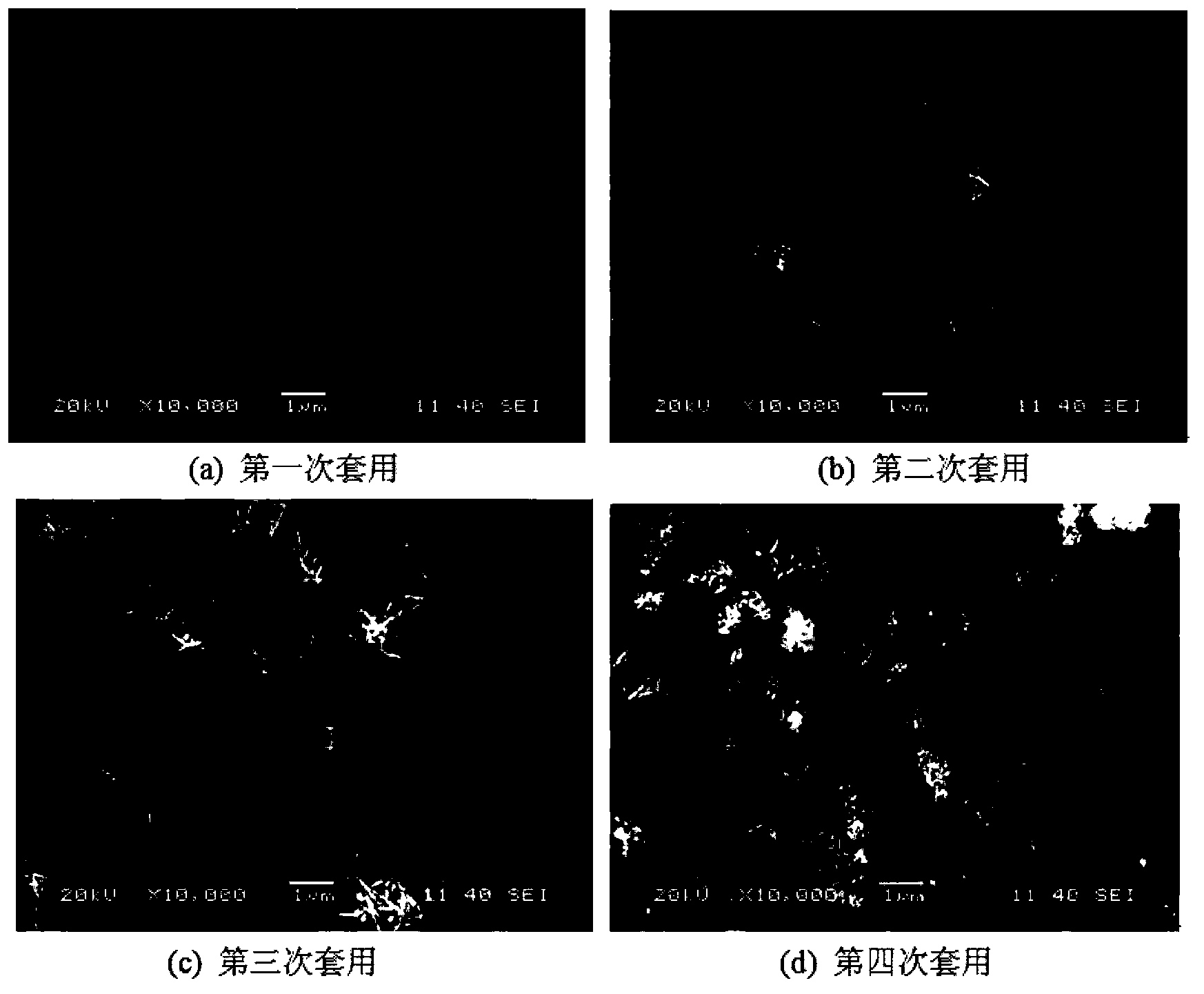
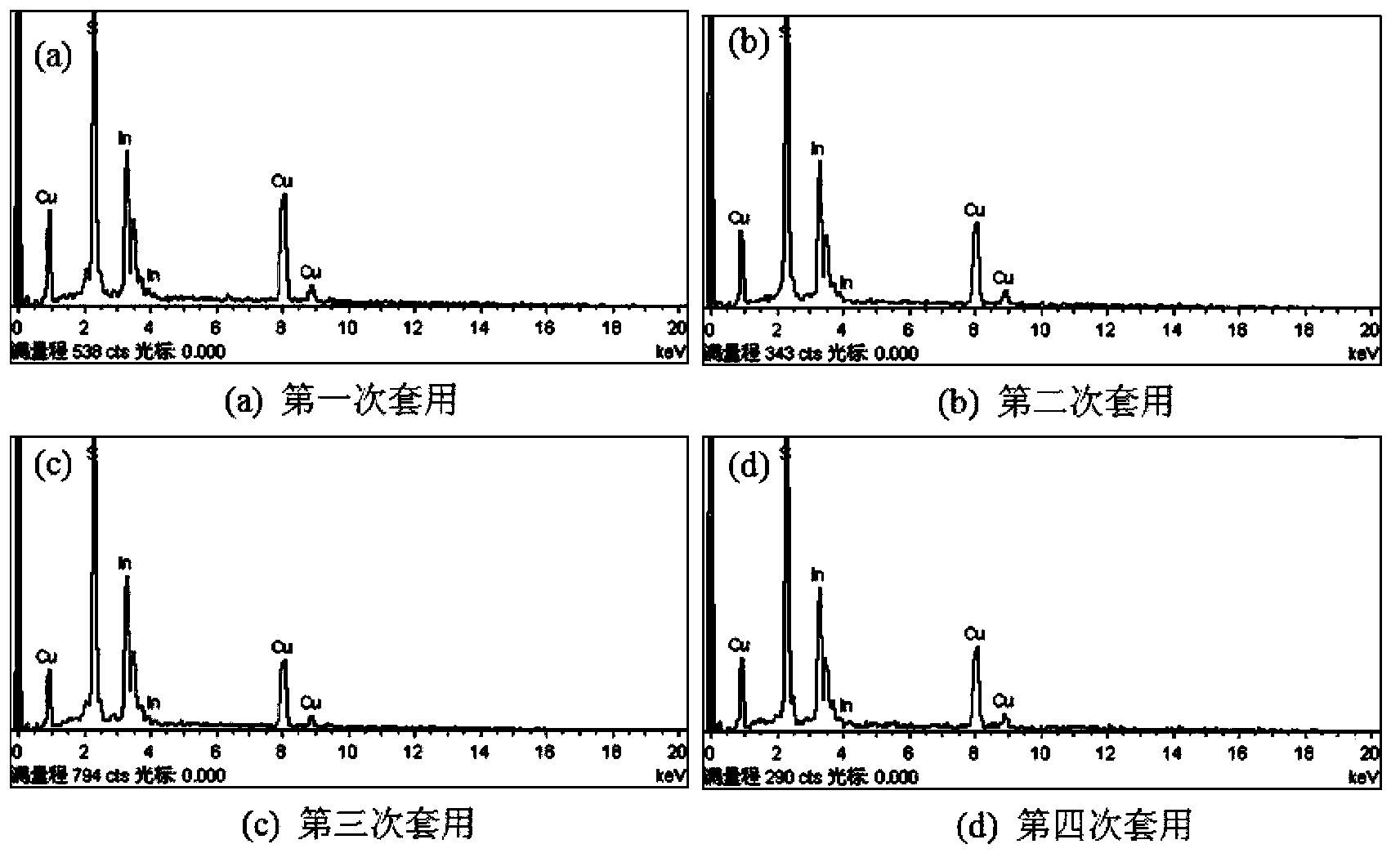
Method for preparing CuInS2 nanocrystals by using mother liquor through solvothermal process
A technology of solvothermal method and mother liquor, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve the problems of large solvent discharge, unfavorable low-carbon environmental protection, etc., and reduce waste liquid discharge and treatment, high solid relative yield, and suitable band gap width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Weigh 0.4651g CuCl with an analytical balance 2 2H 2 O, 0.8000g InCl 3 4H 2 O, 0.5192gCH 4 N 2 S, successively placed in 40ml N,N-dimethylformamide (DMF), stirred to fully dissolve it, and a yellow clear solution was obtained. where n(CuCl 2 2H 2 O):n(InCl 3 4H 2 O):n(CH 4 N 2 S)=1︰1︰2.5.
[0023] 2) Measure 40ml of ethylene glycol (EG) and slowly pour it into the solution in step 1), stir to disperse it evenly, and obtain a colorless and clear solution. Add concentrated hydrochloric acid to adjust the pH of the system to 2-3.
[0024] 3) Transfer the mixed solution in step 2) into a polytetrafluoroethylene-lined autoclave with a volume of 100mL and seal it, react at 190°C for 12h, and then cool down to room temperature naturally.
[0025] 4) Centrifuge the reaction feed liquid in step 3) in an electric centrifuge for 15 minutes at a speed of 3000 r / min, then pour out the upper layer and clear it for later use. The samples were washed several times with...
Embodiment 2
[0027] 1) Weigh 0.4651g CuCl with an analytical balance 2 2H 2 O, 0.8000g InCl 3 4H 2 O, 0.5192gCH 4 N 2 S, sequentially placed in the first solid-liquid separation mother liquor in Example 1, stirred to make it evenly dispersed.
[0028] 2) Add concentrated hydrochloric acid to the mixed solution in step 1) to adjust the pH of the system to 2-3.
[0029] 3) Transfer the mixed solution in step 2) into a polytetrafluoroethylene-lined autoclave with a volume of 100mL and seal it, react at 190°C for 12h, and then cool down to room temperature naturally.
[0030] 4) Centrifuge the reaction feed liquid in step 3) in an electric centrifuge for 15 minutes at a speed of 3000 r / min, then pour out the upper layer and clear it for later use. The samples were washed several times with absolute ethanol and deionized water. The obtained product was dried in a vacuum oven at 60° C. for 6 hours, and a sample was taken out to obtain a black solid powder, which was weighed and packaged. ...
Embodiment 3
[0032] 1) Weigh 0.4651g CuCl with an analytical balance 2 2H 2 O, 0.8000g InCl 3 4H 2 O, 0.5192gCH 4 N 2 S, sequentially placed in the first solid-liquid separation mother liquor in Example 2, stirred to make it evenly dispersed.
[0033] 2) Add concentrated hydrochloric acid to the mixed solution in step 1) to adjust the pH of the system to 2-3.
[0034] 3) Transfer the mixed solution in step 2) into a polytetrafluoroethylene-lined autoclave with a volume of 100mL and seal it, react at 190°C for 12h, and then cool down to room temperature naturally.
[0035] 4) Centrifuge the reaction feed liquid in step 3) in an electric centrifuge for 15 minutes at a speed of 3000 r / min, then pour out the upper layer and clear it for later use. The samples were washed several times with absolute ethanol and deionized water. The obtained product was dried in a vacuum oven at 60° C. for 6 hours, and a sample was taken out to obtain a black solid powder, which was weighed and packaged. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com